
- •Preface
- •Acknowledgments
- •Contents
- •Contributors
- •1. Introduction
- •2. Evaluation of the Craniomaxillofacial Deformity Patient
- •3. Craniofacial Deformities: Review of Etiologies, Distribution, and Their Classification
- •4. Etiology of Skeletal Malocclusion
- •5. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Traumatic Defects
- •6. Etiology, Distribution, and Classification of Craniomaxillofacial Deformities: Review of Nasal Deformities
- •7. Review of Benign Tumors of the Maxillofacial Region and Considerations for Bone Invasion
- •8. Oral Malignancies: Etiology, Distribution, and Basic Treatment Considerations
- •9. Craniomaxillofacial Bone Infections: Etiologies, Distributions, and Associated Defects
- •11. Craniomaxillofacial Bone Healing, Biomechanics, and Rigid Internal Fixation
- •12. Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
- •13. Bioresorbable Materials for Bone Fixation: Review of Biological Concepts and Mechanical Aspects
- •14. Advanced Bone Healing Concepts in Craniomaxillofacial Reconstructive and Corrective Bone Surgery
- •15. The ITI Dental Implant System
- •16. Localized Ridge Augmentation Using Guided Bone Regeneration in Deficient Implant Sites
- •17. The ITI Dental Implant System in Maxillofacial Applications
- •18. Maxillary Sinus Grafting and Osseointegration Surgery
- •19. Computerized Tomography and Its Use for Craniomaxillofacial Dental Implantology
- •20B. Atlas of Cases
- •21A. Prosthodontic Considerations in Dental Implant Restoration
- •21B. Overdenture Case Reports
- •22. AO/ASIF Mandibular Hardware
- •23. Aesthetic Considerations in Reconstructive and Corrective Craniomaxillofacial Bone Surgery
- •24. Considerations for Reconstruction of the Head and Neck Oncologic Patient
- •25. Autogenous Bone Grafts in Maxillofacial Reconstruction
- •26. Current Practice and Future Trends in Craniomaxillofacial Reconstructive and Corrective Microvascular Bone Surgery
- •27. Considerations in the Fixation of Bone Grafts for the Reconstruction of Mandibular Continuity Defects
- •28. Indications and Technical Considerations of Different Fibula Grafts
- •29. Soft Tissue Flaps for Coverage of Craniomaxillofacial Osseous Continuity Defects with or Without Bone Graft and Rigid Fixation
- •30. Mandibular Condyle Reconstruction with Free Costochondral Grafting
- •31. Microsurgical Reconstruction of Large Defects of the Maxilla, Midface, and Cranial Base
- •32. Condylar Prosthesis for the Replacement of the Mandibular Condyle
- •33. Problems Related to Mandibular Condylar Prosthesis
- •34. Reconstruction of Defects of the Mandibular Angle
- •35. Mandibular Body Reconstruction
- •36. Marginal Mandibulectomy
- •37. Reconstruction of Extensive Anterior Defects of the Mandible
- •38. Radiation Therapy and Considerations for Internal Fixation Devices
- •39. Management of Posttraumatic Osteomyelitis of the Mandible
- •40. Bilateral Maxillary Defects: THORP Plate Reconstruction with Removable Prosthesis
- •41. AO/ASIF Craniofacial Fixation System Hardware
- •43. Orbital Reconstruction
- •44. Nasal Reconstruction Using Bone Grafts and Rigid Internal Fixation
- •46. Orthognathic Examination
- •47. Considerations in Planning for Bimaxillary Surgery and the Implications of Rigid Internal Fixation
- •48. Reconstruction of Cleft Lip and Palate Osseous Defects and Deformities
- •49. Maxillary Osteotomies and Considerations for Rigid Internal Fixation
- •50. Mandibular Osteotomies and Considerations for Rigid Internal Fixation
- •51. Genioplasty Techniques and Considerations for Rigid Internal Fixation
- •52. Long-Term Stability of Maxillary and Mandibular Osteotomies with Rigid Internal Fixation
- •53. Le Fort II and Le Fort III Osteotomies for Midface Reconstruction and Considerations for Internal Fixation
- •54. Craniofacial Deformities: Introduction and Principles of Management
- •55. The Effects of Plate and Screw Fixation on the Growing Craniofacial Skeleton
- •56. Calvarial Bone Graft Harvesting Techniques: Considerations for Their Use with Rigid Fixation Techniques in the Craniomaxillofacial Region
- •57. Crouzon Syndrome: Basic Dysmorphology and Staging of Reconstruction
- •58. Hemifacial Microsomia
- •59. Orbital Hypertelorism: Surgical Management
- •60. Surgical Correction of the Apert Craniofacial Deformities
- •Index
12
Metal for Craniomaxillofacial Internal Fixation Implants and Its Physiological Implications
Samuel G. Steinemann
Implants function as a temporary splint. In the form of a screw, a plate, or a pin, the implant stabilizes the fracture and supports forces in addition to those of functional load. Yet the implant is a foreign body. Is this foreign body an insult of the chemical, physiological, or mechanical kind for the living tissue?
Simpson et al.1 made a comprehensive study of fracturetreatment implants of stainless steel, cobalt-base alloy, and titanium. Clinical symptoms of pain, swelling, and inflammation are observed with the first two metals but none with titanium. Local findings show sequestration for stainless steel and cobalt-base alloy implants, and inertness (i.e., absence of infection and a loose, vascularized tissue in contact with titanium implants (Figure 12.1). Such observations certainly distinguish the “2nd generation metal” titanium from the classical materials stainless steel and cobalt-chromium- molybdenum alloy.
Corrosion and Tissue Reaction
In the chemist’s view, corrosion is the visible destruction of a metal. It may cause rupture of a structure or loss of function (e.g., by breakage of an implant). That was before the 1960s. This aspect is not important for modern metals in surgery because an attack is so small that a material loss is neither visible nor can it be weighed. More sensitive electrochemical methods are needed to measure corrosion, but experiments that reproduce the real conditions for a surgical implant in tissue are not simple.2,3
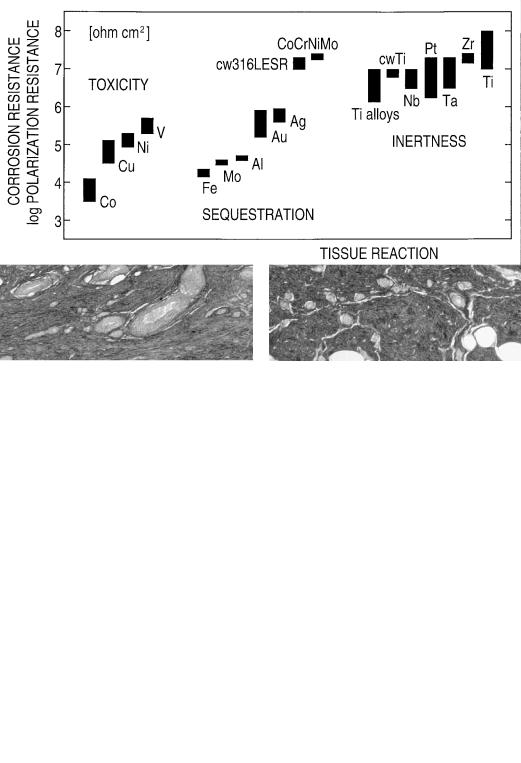
The polarization resistance method has been used for in vivo experiments,2 with results shown in Figure 12.2. The method requires minimally invasive procedures and is characterized by reduction and oxidation reactions on the metal that are not forced and run freely.
The noble metals silver (Ag) and gold (Au) have a resistance to corrosion, lying about in the middle of the scale of the corrosion resistance. In the logarithmic scale of the polarization resistance, the number is 5 to 6, thus about 2 units or
a factor of 100 lower than high-grade stainless steel and titanium. Gold and silver do resist oxidation in air, but are much less resistant to corrosion in sea water and biological fluids. It is common experience that they lose polish after some time.
Metals having lower corrosion resistance than silver and gold (e.g., aluminum, molybdenum, and iron) show visible attack or oxidation in living tissue, and they are always surrounded by a pseudomembrane (i.e., such metals are sequestrated; however, without manifest pathological changes). This tissue reaction equals to a chemical insult. In fact, the group of metals iron (Fe) through silver corrodes so rapidly that supply and migration of oxygen cannot follow the consumption of the oxidant, so that the tissue starves of oxygen. This direct effect of corrosion is not specific for the metal of the implant. However, metals are released with the corrosion process and some of them are cell-toxic. Gerber et al.4,5 and Rae6 measured the toxicity by adding metal salts to embryonic bone rudiments and fibroblast cultures and observing inhibition reactions. Among the four elements shown in Figure 12.2, vanadium (V) is the most toxic and copper (Cu) the least toxic. This reaction equals to a major physiological insult.
The polarization resistance of stainless steel, cobalt-base alloy, and titanium is about the same for all three metals, but tissue reactions differ (see Figure 12.1). High corrosion resistance is apparently not sufficient to suppress a minor rejection reaction observed for the two classical alloys, which include cell-toxic nickel (Ni) and cobalt (Co) as essential components.
Fate of the Unwanted Reaction Product of
Corrosion—Physiological Insult
The species of metal compounds ingested with nutrition and making the passage in the bloodstream (i.e., being metabolized) as well as those finally stored in organs and tissue are rather incompletely known.7 However, most metals (other than alkali) in body fluids and tissue are bound to organic matter and exist in a stable, electrically uncharged form.
107
108 |
S.G. Steinemann |
a |
b |
FIGURE 12.1 Optical micrographs of tissue in contact with a stainless steel (a) and a commercially pure titanium implant (b). Biopsies are taken at metal retrieval about 1.5 years after operation. Blood
and lymphatic vessels are seen throughout the contact zone for titanium but not in the case of stainless steel.
FIGURE 12.2 Data from in vivo corrosion experiments for various metallic elements and for practical alloys. The diagram has the following two coordinates: tissue reaction as abscissa, and polarization/corrosion resistance as the ordinate. Tissue reaction is grouped according to the three distinct forms of toxicity, sequestration, and inertness. Corrosion resistance is roughly proportional to the measured
polarization resistance. It is noted that the useful scale in chemistry and biology is always the logarithmic one; thus differences over the series, for example, from cobalt (Co) to silver (Ag) to titanium (Ti), amount to factors of 1 to 100 to 10,000 in corrosion resistance. CoCrNiMo is wroughtcobalt-basealloy,cw316LESRiscold-worked,remeltedstain- less steel, Ti alloys are Ti4Al4Mo, Ti6Al4V, and Ti15Mo.

12. Metal for Craniomaxillofacial Internal Fixation Implants
FIGURE 12.3 A sketch intended to illustrate that metals released from implants follow another reaction path than metals entering metabolism with nutrition.
Figure 12.3 suggests that metal release from implants involves a different path. It can be associated with the entry of the metal through a wound, which then undergoes corrosion. The unwanted reaction products of this corrosion are hydroxides, hydrous oxides, and oxides (i.e., sparingly soluble salts and occasionally complexes such as halides). These salts can be soluble or not in the tissue fluids (which are aqueous electrolytes), and they can be toxic or not.8 To know the effects of this corrosion burden, the identity and stability of the hydrolysis products must be considered.
109
The word hydrolysis is applied to chemical reactions in which a substance is split or decomposed by water. What are the conversions of the metal salt, and does hydrolysis involve electrically neutral and ionic species, either the positively charged cation, the negatively charged anion, or both? These questions are addressed for two metallic biomaterials, stainless steel, with nickel as the main component, and titanium.
It is common to find that salts and oxides dissolve easily in strong acids and in strong bases and that the solubility of oxides is low around neutral pH values. Further, oxides dissociate and cations, either as bare ions or an ion comprising the hydroxide as ligand, dominate at low pH, while anions (always comprising the hydroxide ligand) exist at high pH. In between, uncharged aqueous species exist but must not dominate. Measurements of the kind are best represented as the solubility, or distribution curves of the various hydrolysis products. Diagrams have the two coordinates, acidity (i.e., pH of the solution) as abscissa and molar concentration of dissolved and precipitated species as the ordinate. Both are in logarithmic scale, which gives the straight lines.
The 2 oxidation state of nickel is the important one, and in an aqueous environment the hydroxide is the first-formed corrosion product. Its dominant hydrolysis product under physiological conditions is the unhydrolyzed nickel cation with a concentration of about 1 mmol at the limit of hydroxide precipitation (Figure 12.4). The unwanted reaction product of corrosion is an ion.
In serum, the nickel concentration is about 10 nM, and in human skeletal muscle it is about 3 M and less than the solubility limit. The metal concentration in the contact tissue around implants is still 100 times higher than that of normal muscle tissue and of the order of the toxicity threshold for nickel. The sequestration reaction for stainless steel implants is the consequence.
FIGURE 12.4 Distribution of hydrolysis products in solutions saturated with respect to nickel hydroxide [Ni(OH)2]. The full line is the solubility limit expressed as the total concentration of two-valent Ni. Data for the concentration of nickel in serum (S), in muscle (M), in
contact tissue around stainless steel implants (I), and toxicity levels
(T) are added at right margin. Hydrolysis results are from Baes and Mesmer,9 tissue concentrations of Ni are collected from many sources in Steinemann,10 and toxicity levels are from Gerber et al.4,6 and Rae.6

110
FIGURE 12.5 Solubility behavior of hydrous titanium-dioxide measured in sodium chloride and chlorate electrolytes.9,10 The full line is the solubility limit and dashed lines are partial concentrations for
Titanium is a reactive metal. In air and electrolytes, it forms spontaneously a dense and electrically insulating oxide film at its surface. The unwanted reaction product becomes a potent barrier against dissolution of the metal.
The constant solubility of titanium dioxide above a pH of around 3 and up to a pH of around 12 suggests that an electroneutral species dominates in solution (Figure 12.5). At physiological pH values, the first charged species is the cation Ti(OH)3 with a concentration of not more than 0.1 nM, which is by orders of magnitude lower than the concentration of the always-present hydrogen ion in solution. The unwanted reaction product of corrosion is not an ion. This is an important finding because uncharged hydrolysis products have no affinity for reaction with organic molecules. Corrosion of titanium becomes, in fact, no chemical burden and its inert reaction in tissue is a sign of the basically different chemistry in solution.
In serum, the titanium concentration is about 0.1 M, and in human skeletal muscle, it is about 5 M. The muscle concentration of titanium equals the upper limit of solubility for the aqueous hydroxide, which is also the lower limit for precipitation of the solid oxide (about 3 M). Solution chemistry thus provides a stringent, even simple homeostatic mechanism for the regulation of titanium in tissue: titanium is at saturation in tissue. The concentration of titanium in the contact tissue around implants is about 300 times higher than that of muscle tissue. These high concentrations seem representative for larger and loaded implants and include fretting and wear debris and residues from insufficient surface treatment. These metal and oxide particles beyond the solubility limit are deposited in tissue. Retrieval studies give no indication of any adverse reaction.
The in vivo corrosion experiments led us to distinguish for three forms of local tissue reaction (see Figure 12.2). The unwanted reaction product of corrosion is the cause. Tissue impregnation by corrosion products equals to a chemical insult, but it is crucial to ask whether it is an ion or an uncharged inorganic compound. With this distinction, toxicity is a major
S.G. Steinemann
the named species. Data for the concentration of titanium in serum (S), in muscle (M), and in contact tissue around implants (I) are added at right margin (numerous sources10).
physiological insult, and sequestration is a minor physiological insult, while inertness equals to no physiological insult.
The distinction between ions and uncharged inorganic compounds has a prolongation for immunologic reactions. Metals can act as haptens, that is, the ion can unite with a protein to form an antigen.7,11 Such complex formation is known to occur with ions of nickel, cobalt, and chromium, but it is absent for titanium and a few other metals whose hydrolysis products in tissue fluids are not ions.
Note that no case of local or systemic reaction for titanium is documented.
Titanium has the surprising property that it can bind to living tissue and to bone. Dental surgeons use this quality for dental implants and call it osseointegration. The binding between the metal and bone resists forces along the interface (shear) and the perpendicular to the interface (tear off) and the adhesion is quite strong.12 What is the glue? It is a true bonding interaction between hydroxyls in the ever-present surface titanium dioxide and the various ligands of organic matter.13 These basic processes occur in atomic dimensions, and Listgarten14 notes in his high-resolution transmission electron microscopic pictures that “there is no evidence of any space between the metallic surface and the bone.” The affinity between bone and titanium may be termed pseudobiological activity.
Metal and Implants—Mechanical
Properties and Manufacture
Titanium is not a rare metal, but its reduction from ore (ilmenite, an iron-titanium-oxygen compound, and rutile, an oxide of titanium) is not easy and in industrial scale succeeded only in the 1940s. The metal is used for aircraft, in chemical industry, and since the 1960s for surgical implants. Specifications for the application exist today.
AO/ASIF implants for craniomaxillofacial bone surgery are made from commercially pure (cp) titanium, with the excep-

12. Metal for Craniomaxillofacial Internal Fixation Implants
TABLE 12.1 Mechanical properties of stainless steel, titanium, and bone.
|
Yield |
|
Young’s |
Material |
strength, MPa |
Ductility, % |
modulus, GPa |
|
|
|
|
Cold-worked stainless steel |
730 |
21% |
190 |
cp titanium (grade 2) |
280 |
(30%) |
105 |
Cold-worked Ti (grade 4) |
690 |
18% |
105 |
Cortical bone |
140 |
|
18 |
|
|
|
|
tion of a mandibular reconstruction set in stainless steel. Oxygen is added in small amounts, which increases the mechanical strength (Table 12.1). Grade 2 metal is used when malleability is more important than strength (thin bone, midface), while screws and loaded implants (mandible region) are made from the stronger, cold-worked metal. Shapes are obtained by machining (cutting, milling, drilling) operations.
Implants for head surgery must cause the smallest mechanical insult; a minimum volume and a smooth form is required (Figure 12.6). Titanium is the indicated material. It has good strength and high admissible strain to avoid overload of the implant. This admissible strain, equal to the ratio yield strength divided by Young’s modulus, is 7% for cold-worked titanium, higher than that of stainless steel (4%), and about equal to that of bone (8%).
The last steps in fabrication are mechanical and chemical surface treatments. These processes augment the inertness of the implant. The matte yellow surface results from pickling in acid and the anodic oxidation that makes an implant inconspicuous in tissue and near the skin. This surface treatment further stabilizes an osteosynthesis. Experiments show that the release torque of small bone screws exceeds the in-
a
111
sertion torque after 3 months.15 Stainless steel screws, on the the contrary, always loosen with time.
Clinical Implications
Titanium implants are fully inert in tissue, and screws made of titanium integrate for a “solid mounting” of the osteosynthesis. Such behavior could suggest that we should “fit and forget” the fracture implant. The proposition is restricted to titanium and does not apply for stainless steel implants.
Head trauma, reconstructive, and corrective surgery can require large implants or a great number of small implants, sometimes in conjunction with dental implants to achieve functional rehabilitation. Some questions may emerge:
Is there a limit to the number or the surface area of implants that can be placed? Experience indicates no restrictions, based on the absence of foreign body reactions and the fact that living tissue is saturated with titanium. A larger size or greater number of implants is not a burden.
Is there an interaction among several implants? The answer is no. Mutual interaction between two plates or with a dental implant would at least require electrical contact.
Can another kind of insult occur? A bad mechanical situation may exist if solid mounting (i.e., stability of the osteosynthesis) is not achieved. Displacement by too much metal is a mechanical insult. Displacement can also limit indications for operations on children and adolescents.
Indications for removal of the implants do exist. Because of their favorable chemical and biochemical properties, titanium integrates easily and rapidly, especially for children. Thus removal should be done early.
b
FIGURE 12.6 Smooth and malleable shapes with minimum volume are important for internal fixation implants in maxillofacial surgery. Fixation of a fractured mandible by two miniplates (a). Postopera-
tive view of fracture fixation by a miniplate on the superior border of the mandibular angle (b). (Courtesy STRATEC Medical, Oberdorf, Switzerland)
112
References
1.Simpson JP, Geret V, Brown SA, Merritt K. Retrieved fracture plates—implant and tissue analysis. In: Weinstein A, Gibbons D, Brown S, Ruoff S, eds. Implant Retrieval—Material and Biological Analysis. NBS Spec. Publ. 601, 1981:395–422.
2.Steinemann SG. Corrosion of titanium and titanium alloys for surgical implants. In: Lütjering G, Zwicker V, Bunk W, eds. Titanium, Science and Technology; Proc 5th Intl Conf Titanium.
Oberursel: Deutsche Gesellschaft Metallkunde. 1985:1373– 1379.
3.Steinemann SG. Corrosion of implant alloys. In: Buchhorn GH, Willert HG, eds. Technical Principles, Design and Safety of Joint Implants. Seattle: Hogrefe & Huber Publishers; 1994: 168–179.
4.Gerber H, Perren SM. Evaluation of tissue compatibility of in vitro cultures of embryonic bone. In: Winter GD, Leray JL, de Groot K, eds. Evaluation of Biomaterials. Chichester: John Wiley & Sons, 1980:307–314.
5.Gerber HW, Moosmann A, Steinemann S. Bioactivity of met- als—tissue tolerance of soluble or solid metal, tested on organ cultured embryonic bone rudiments. In: Buchhorn GH, Willert HG, eds. Technical Principles, Design and Safety of Joint Implants. Seattle: Hogreve & Huber Publishers; 1994:248–254.
6.Rae T. The toxicity of metals used in orthopaedic prostheses. J Bone Joint Surg. 1981;63-B:435–440.
7.Luckey TD, Venugopal B. Metal Toxicity in Mammals, Vol. 1, Physiological and Chemical Basis. New York: Plenum Press, 1977:39–91, 103–128.
8.Steinemann SG, Mäusli P-A. Titanium alloys for surgical im-
S.G. Steinemann
plants—biocompatibility from physicochemical principles. In: Lacombe P, Tricot R, Béranger G, eds. 6th World Conf Titanium, France 1988. Les Ulis: Les éditions de physique. 1988; 535–540.
9.Baes CF, Mesmer RE. The Hydrolysis of Cations. New York: John Wiley & Sons; 1976.
10.Steinemann SG. Tissue compatibility of metals from physicochemical principles. In: Kovacs P, Istephanous NS, eds. Proc Symp Compatibility of Biomedical Implants, vol. 94-15. Pennington, NJ: The Electrochemical Society, 1994:1–13.
11.Black J. Biological Performance of Materials. New York: Marcel Dekker; 1992:184–199.
12.Steinemann SG, Eulenberger J, Mäusli P-A, Schroeder A. Adhesion of bone to titanium. In: Christel P, Meunier A, Lee AJC, eds. Biological and Biomechanical Performance of Biomaterials. Amsterdam: Elsevier Science Publishers. 1986:409–414.
13.Gold JM, Schmidt M, Steinemann SG. XPS study of amino acid adsorption to titanium surfaces. Helv Phys Acta. 1989:62: 246–249. Idem. XPS study of retrieved titanium and Ti alloy implants. In: Heimke G, Soltész V, Lee AJC, eds. Clinical Implant Materials—Advances in Biomaterials, vol. 9. Amsterdam: Elsevier Science; 1990:69–74.
14.Listgarten MA, Buser D, Steinemann SG, Donath K, Lang NP, Weber H-P. Light and transmission electron microscopy of the intact interfaces between non-submerged titanium-coated epoxy resin implants and bone or gingiva. J Dental Res. 1992; 71: 364–371.
15.Eulenberger J, Steinemann SG. Lösemomente an Kleinschrauben aus Stahl und Titan mit unterschiedlichen Oberflächen. Unfallchirurg. 1990;93:96–99.
