
Principles and Applications of Asymmetric Synthesis
.pdf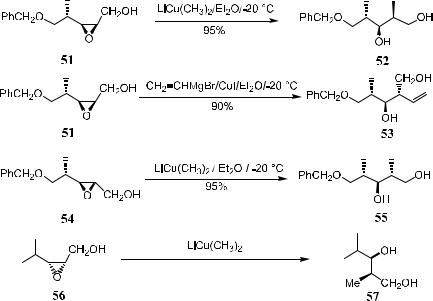
|
|
4.2 |
SELECTIVE OPENING OF 2,3-EPOXY ALCOHOLS |
211 |
|||||
TABLE 4±6. LiBH4 Reduction of 2,3-Epoxy Alcohols |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
Ti(OR)4 |
|
|
Condition |
|
Ratio |
|
|
|
|
|
|
|
|
|
|
||
Entry |
Substrate |
|
Solvent Temp. ( C) Time |
|
|
||||
eq. |
|
1,2-:1,3- |
Yield (%) |
||||||
1 |
49a |
1.5 |
THF |
65 |
1 h |
7.3:1 |
|
83 |
|
2 |
49b |
1.9 |
C6H6 |
10 |
20 h |
145:1 |
|
93.2 |
|
3 |
49c |
1.7 |
C6H6 |
50 |
15 min |
46:1 |
|
99 |
|
4 |
49c |
1.7 |
C6H6 |
10 |
18 h |
150:1 |
|
97 |
|
5 |
50 |
1.5 |
THF |
65 |
15 min |
1.7:1 |
|
84.3 |
|
6 |
50 |
1.6 |
C6H6 |
50 |
45 min |
6.8:1 |
|
96.3 |
|
Reprinted with permission by Pergamon-Elsevier Science Ltd., Ref. 33.
Scheme 4±18 shows, epoxides 51, 54, and 56 can be converted to the corresponding 1,3-diols 52, 53, 55, and 57 in high yield and ee through dialkylcuprate treatment.35
Scheme 4±18. Ring opening by dialkylcuprate.
4.2.5Payne Rearrangement and Ring-Opening Processes
Thus far, we have discussed nucleophilic ring opening in 2,3-epoxy-1-ol taking place at the C-2 and C-3 positions (see compound 58 in Scheme 4±19). However, in the presence of a base, nucleophilic ring opening can take place at C-1 via Payne rearrangement to produce 2,3-diol.36 For example, compound 1,2-

212 ASYMMETRIC OXIDATIONS
Scheme 4±19. Payne rearrangement and subsequent ring opening.
Scheme 4±20. PhSÿ as a nucleophile in the preparation of tetritol precursors.
epoxy-3-ol 59 was ®rst produced via this rearrangement. Subsequent ring opening at C-1 gives the corresponding 2,3-diol 60 (Scheme 4±19). Various nucleophiles such as PhSÿ, BH4ÿ, CNÿ, and TsNHÿ can be used for this purpose.
Scheme 4±20 exempli®es PhSÿ attack mediated by a Payne rearrangement. The selective ring-opening product can be applied to prepare tetritols.37
This approach provides a new method for carbohydrate synthesis. In the synthesis of tetritols, pentitols, and hexitols, for example, titanium-catalyzed asymmetric epoxidation and the subsequent ring opening of the thus formed 2,3-epoxy alcohols can play an essential role.
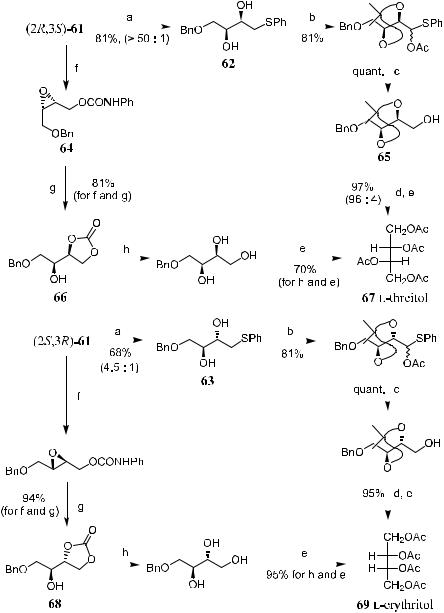
Scheme 4±21 shows the preparation of l-threitol and l-erythritol.38 Epoxy alcohols …2R,3S†-61 and …2S,3R†-61, generated by asymmetric epoxidation, are exposed to sodium benzenethiolate and sodium hydroxide in a protonic solvent to undergo base-catalyzed rearrangement, yielding the threo-diol 62 and erythrodiol 63, which can then be converted to the corresponding tetraacetate of l- threitol 67 and l-erythritol 69 through subsequent transformations.
Another approach to l-threitol 67 and l-erythritol 69 is stereoselective ring opening at the C-2 position of …2R,3S†-61 using intramolecular oxygen as the nucleophile. With the aid of an acid catalyst, compound phenylurethane 64 undergoes ring opening to give carbonate 66, which can then be converted to the known tetraacetate 67 via routine chemistry. Similarly, compound 69 can be obtained from …2S,3R†-61, which furnishes a synthesis of l-threitols and l-erythritols.
|
|
|
|
|
|
4.2 SELECTIVE OPENING OF 2,3-EPOXY ALCOHOLS |
213 |
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±21. Asymmetric synthesis of tetritol isomers 67 and 69. Reagents and conditions: a: NaOH, PhSH (dioxane, H2O), 65 C, 3 h. b: (1) Me2C(OMe)2, H‡; (2) m- CPBA, CH2Cl2, ÿ20 C, 1 h; (3) Ac2O, NaOAc, re¯ux, 6 h. c: LAH, ether, 0 C, 1 h. d: MeOH, H‡, 70 C, 1 h. e: (1) H2, Pd/C, acidic MeOH, 25 C, 6 h; (2) Ac2O, C5H5N. f: PhNCO, (Et)3N, CH2Cl2, 25 C, 24 h. g: 5% HClO4, CH3CN, 25 C, 24 h. h: NaOH, aq. MeOH, 25 C, 24 h.
214 ASYMMETRIC OXIDATIONS
4.2.6Asymmetric Desymmetrization of meso-Epoxides
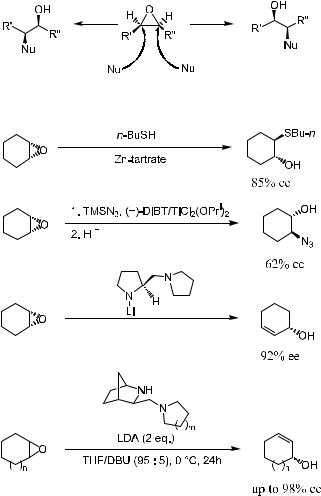
Complementary to the regioselective and stereoselective ring opening of epoxides, desymmetrization of meso-epoxides by oxirane-ring opening with nucleophiles in an asymmetric manner, as shown in Scheme 4±22, should also be mentioned. In the presence of certain Lewis acids, the metal center of a catalyst or reagent is able to coordinate to the epoxide oxygen atom. The chiral environment provided by the Lewis acid will then allow an appropriate achiral nucleophile to discriminate the formal enantiotropic carbon±oxygen bond of the epoxide.
Scheme 4±23 presents several examples39 in which the desymmetrization takes place with moderate to excellent enantioselectivity.
Scheme 4±22. Enantioselective ring opening of meso-epoxides …R0 ˆ R00†.
Scheme 4±23. Asymmetric ring opening of meso-epoxides.
4.2 SELECTIVE OPENING OF 2,3-EPOXY ALCOHOLS |
215 |
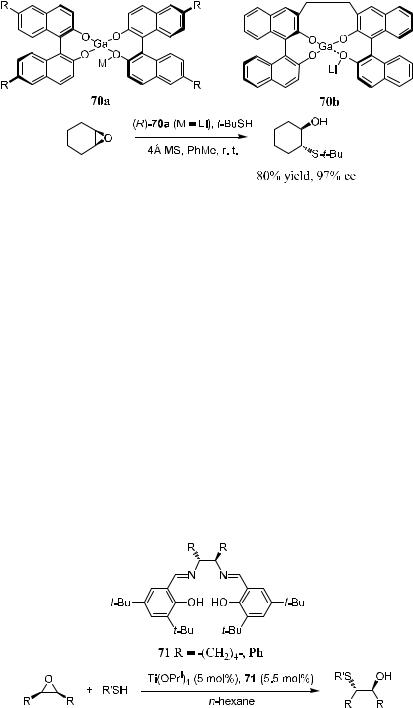
Scheme 4±24. Gallium±lithium complex-catalyzed ring opening.
Iida et al.40 presented gallium complexes 70a and 70b containing chiral BINOL ligand. These complexes have been applied to catalyze the highly enantioselective ring opening of epoxides with thiols or phenols. Scheme 4±24 is an example of using compound 70a as the catalyst for asymmetric ring opening of cyclohexene oxide by t-BuSH. The product can be obtained in good yield and excellent ee. Compound 70a can also be used for enantioselective ring opening of epoxides with 4-methoxyphenol, providing 1,2-diol monoethers with good yields and moderate ee.41 Following his success with this desymmetrization ring opening reaction, Shibasaki then introduced another complex, 70b, in which two binaphthyl ligands are connected. This complex can also be used as a catalyst for phenol-mediated ring-opening reactions.42
The enantioselective ring opening of meso-epoxides with thiols can also be facilitated by chiral (salen)Ti(IV) complex.43 As shown in Scheme 4±25, in the presence of salen compound 71 and Ti(OPri)4, ring opening of meso-epoxide proceeds at ÿ25 to ÿ40 C, giving a product with good chemical yield and moderate ee.
Salen±transition metal complex, or Jacobsen reagent, has been found useful in a range of asymmetric reactions, such as Diels-Alder reactions or the epox-
Scheme 4±25
216 ASYMMETRIC OXIDATIONS
Scheme 4±26
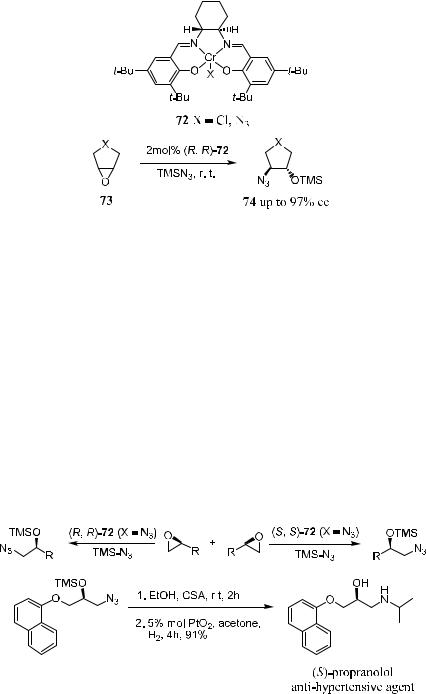
idation of unfunctionalized ole®ns. (These reactions are discussed later.) Schaus et al.44 have reported a practical synthesis of enantiomerically pure cyclic 1,2- amino alcohols via catalytic asymmetric ring opening of meso-epoxides. As shown in Scheme 4±26, reagent 72 catalyzes azide attack on the epoxide 73, resulting in chiral product 74 in high ee. From 74, a series of 1,2-amino alcohols can be prepared. For example, 72 (X ˆ Cl)±catalyzed reactions give ee of 94%, 88%, 95%, and 97% for product 74 where X ˆ CH2, (CH2)2, NCOCF3, and O, respectively.
Jacobsen and colleagues45 also report applying the (salen)Cr±N3-catalyzed epoxide ring-opening reaction in the kinetic resolution of racemic terminal epoxides. Not only can the remaining epoxides be recovered with high ee, but also 1-azido-2-trimethyl siloxyalkanes can be obtained in good yield and very high ee. As an example, the precursor for (S)-propranolol, an antihypertensive agent, can be readily prepared via asymmetric ring opening of the epoxide compound (Scheme 4±27).
Scheme 4±27
4.3 ASYMMETRIC EPOXIDATION OF SYMMETRIC DIVINYL CARBINOLS |
217 |
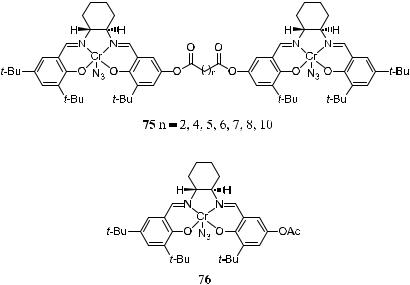
Jacobsen has also designed a dimeric catalyst in which two salen complexes are connected together. A pronounced cooperative catalysis e¨ect is observed, leading to reaction rate enhancement even at a very low catalyst concentration. For example, the rate constant for asymmetric ring opening catalyzed by 75 (n ˆ 5) is two orders of magnitude higher than that catalyzed by the control catalyst 76, while the enantioselectivity is comparable.46 This reaction and the cooperative catalysis are further discussed in Chapter 8.
4.3 ASYMMETRIC EPOXIDATION OF SYMMETRIC DIVINYL CARBINOLS
An important consideration in designing and performing asymmetric reactions with high regioselectivity and enantioselectivity, especially for those in which more than one stereogenic center is formed, is the need to carry out the reaction with a combination of kinetic resolution in the initial asymmetric synthesis.
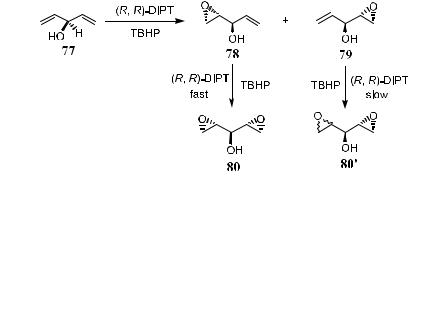
Schreiber et al.47 have described a mathematical model that combines enantiotopic group and diastereotopic face selectivity. They applied the model to a class of examples of epoxidation using several divinyl carbinols as substrates to predict the asymmetric formation of products with enhanced ee (Scheme 4±28).
Consider Sharpless epoxidation with an achiral substrate. With certain ligands, the epoxidation can take place at any one of the four stereotopic faces of the substrate, a¨ording X1, X2, X3, and X4. In Scheme 4±28, X1 reacts fast when A or B is OH, and the reaction is performed in an asymmetric way. When

218 ASYMMETRIC OXIDATIONS
Scheme 4±28. The asymmetric epoxidation of divinyl carbinols. Reprinted with permission by Am. Chem. Soc., Ref. 47b.
a second epoxidation takes place kinetically, the minor enantiomers X2 and X3 proceed faster and are destroyed due to the instability of the diepoxide products Z. Thus, the ratio of X1 to X3 should increase as the reaction proceeds. In other words, the ®rst reaction converts an achiral divinyl carbinol with a pro-stereogenic atom into a chiral nonracemic epoxy alcohol, and the second
4.3 ASYMMETRIC EPOXIDATION OF SYMMETRIC DIVINYL CARBINOLS |
219 |
||||
|
TABLE 4±7. Epoxidation in the Presence of l-(B)-DIPT |
|
|||
|
|
|
|
|
|
|
Conditions |
ee (%) |
de (%) |
|
|
|
|
|
|
|
|
|
3 h, ÿ25 C |
84 |
92 |
|
|
|
24 h, ÿ25 C |
93 |
99.7 |
|
|
|
140 h, ÿ25 C |
V97 |
>99.7 |
|
|
Reprinted with permission by Am. Chem. Soc., Ref. 46b.
reaction occurring in the epoxidation system enhances the ee via a kinetically controlled process.
Three divinyl carbinol substrates have been chosen as examples. They are good substrates for examination because the vinyl carbinols are known to undergo Sharpless reaction at low reaction rates. The results presented in Table 4±7 clearly show that the ee of 79 improves as the reaction proceeds toward completion. Note that the minor enantiomer 78 can be removed through a second, faster epoxidation that converts enantiomer 78 into an easily destroyed bis-epoxide 80 (Scheme 4±29 and Table 4±7). The same trend is apparent in the second demonstration with diisopropenyl carbinol 81 (Scheme 4±30 and Table 4±8). Similarly, the third reaction is the reaction of …E,E†-divinyl carbinol 82 (Scheme 4±31 and Table 4±9).
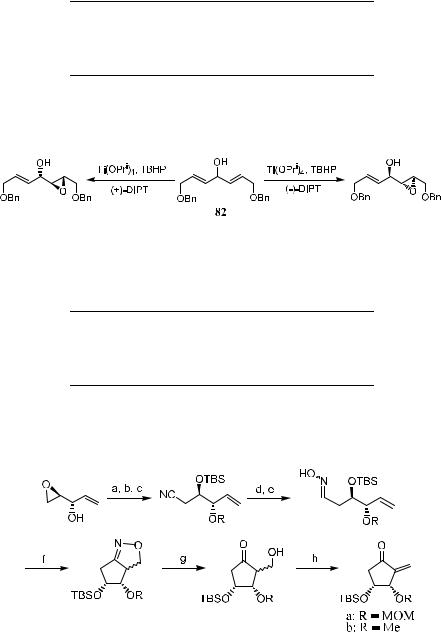
Schreiber's model has been successfully applied to synthesize intermediates of several important natural products, such as prostaglandin intermediate48 (Scheme 4±32) and 2,6-dideoxyhexoses, such as d-(‡)-digitoxose, d-(‡)-cymar- ose, d-(‡)-olivose, and d-(ÿ)-oleandrose49 (see Fig. 4±3 for the structures).
Scheme 4±29
Scheme 4±30. Yield 80±85%.
220 ASYMMETRIC OXIDATIONS
TABLE 4±8. Epoxidation in the Presence of d-(C)-DIPT
Conditions |
ee (%) |
de (%) |
|
|
|
0.5 h, ÿ25 C |
88 |
99 |
1.0 h, ÿ25 C |
94 |
>99 |
1.5 h, ÿ25 C |
>99.3 |
>99 |
de ˆ Diastereomeric excess; ee ˆ enantiomeric excess.
Reprinted with permission by Am. Chem. Soc., Ref. 47b.
Ê
Scheme 4±31. Yield 70±80% without 4 A molecular sieves.
TABLE 4±9. Epoxidation in the Presence of l-(B)-DIPT
Conditions |
ee (%) |
de (%) |
|
|
|
1 h, ÿ25 C |
93 |
V97 |
3 h, ÿ25 C |
95 |
V97 |
44 h, ÿ25 C |
V97 |
V97 |
de ˆ Diastereomeric excess; ee ˆ enantiomeric excess.
Reprinted with permission by Am. Chem. Soc., Ref. 47b.
Scheme 4±32. Highly e½cient synthesis of chiral prostagladin intermediate. Reagents and conditions: a: NaH/MeOCH2Cl or CH3I, 0 C. b: KCN/AcOH. c: TBSCl, imidazole, room temperature. d: DIBAL. e: HONH2 HCl/Py. f: 0.7 N NaOCl/CH2Cl2, room temperature. g: 10% Pd/C, H2. h: MeSO2Cl/Et3N.
