
Principles and Applications of Asymmetric Synthesis
.pdf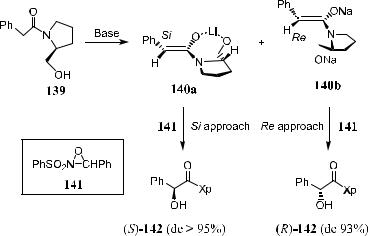
4.8 ASYMMETRIC OXIDATION OF ENOLATES |
251 |
carbonyl group and other related reactions can also be regarded as oxidation reactions. Numerous e¨orts have been made toward the development of a technique for the hydroxylation of a site adjacent to a carbonyl group. Among these, applying chiral N-sulphonyl oxaziridine seems to be a promising approach in a wide variety of enolate hydroxylation reactions.
4.8.1Substrate-Controlled Reactions
In early studies, a-hydroxylation of an adjacent carbonyl group was achieved with control by a chiral auxiliary. This is the case where chiral auxiliaries are used to di¨erentiate the two faces of an enolate, and oxygen transfer produces the corresponding a-hydroxy ketones, esters, or acids after cleavage of the chiral auxiliaries. Representative hydroxylation reactions of chiral enolates include those with substrates derived from metallopyrrolidines, metalloenamines, Oppolzer's sulfonamides, oxazolidinones, and hydrazones. All of these reactions involve a mechanism of diastereofacial discrimination similar to that applied in carbon±carbon bond formation reactions. In this manner, a-hydroxy acid can be obtained from the corresponding chiral enolates derived from pyrrolidinetype imines such as 140a or 140b, when N-sulfonyloxaziridine 141 is used as the oxidant (see Scheme 4±54).109
Scheme 4±54
Evans succeeded in oxidizing N-acyl oxazolidinone enolate 143 or 145 using oxaziridine 141 as the oxidant (Scheme 4±55).110 Representative results are summarized in Table 4±19.

252 ASYMMETRIC OXIDATIONS
Scheme 4±55. N-Acyl oxazolidinone enolate oxidation.
TABLE 4±19. Diasteroselective Hydroxylation of Chiral Carboximide Sodium Enolates Using 2-(Phenylsulfonyl)-3-Phenyl-Oxaziridine (141) in THF at C78 C
Imide |
Yield (%) |
de (%, con®g.) |
||
|
|
|
|
|
143 |
(R ˆ Bn) |
86 |
88 (R) |
|
145a |
(R ˆ Bn) |
85 |
90 (S) |
|
145b |
(R ˆ Bn) |
83 |
90 (S) |
|
143 |
(R ˆ Ph) |
77 |
80 (R) |
|
143 |
(R ˆ Et) |
86 |
88 (R) |
|
143 |
(R ˆ Allyl) |
91 |
90 (R) |
|
143 |
(R ˆ t-Bu) |
94 |
98 (R) |
|
145a |
(R ˆ i-Pr) |
86 |
98 (S) |
|
143 |
(RbMeO2C(CH2)3 |
68 |
92 (R) |
|
de ˆ Diastereomeric excess.
4.8.2Reagent-Controlled Reactions
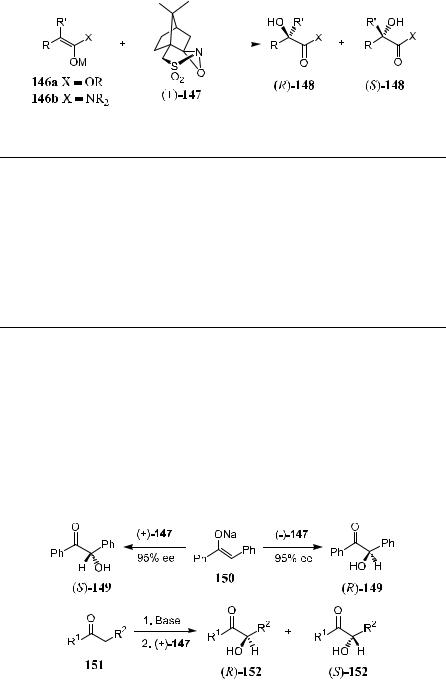
Davis et al.111 developed another method for reagent-controlled asymmetric oxidation of enolates to a-hydroxy carbonyl compounds using (‡)-camphor- sulfonyl oxaziridine (147) as the oxidant. This method a¨orded synthetically useful ee (60±95%) for most carbonyl compounds such as acyclic keto esters, amides, and a-oxo ester enolates (Table 4±20).
4.8 ASYMMETRIC OXIDATION OF ENOLATES |
253 |
||
|
|
|
|
TABLE 4±20. Asymmetric Oxidation of Lithium Enolates and Amides Using (B)-147 as the Oxidant
|
|
Enolate 146 |
|
|
Product 148 |
||
|
|
|
|
|
|
|
|
Entry |
R |
R0 |
X |
Co-solvent |
Temp. ( C) |
Yield (%) |
ee (%) |
1 |
Ph |
H |
t-BuO |
Ð |
ÿ90 |
84 |
71.0(R) |
2 |
Bn |
H |
OMe |
Ð |
ÿ90 |
73 |
58.0(R) |
|
|
|
|
HMPA |
ÿ90 |
63 |
85.5(R) |
3 |
Ph |
H |
OMe |
Ð |
ÿ78 |
84 |
54.0(R) |
4 |
Ph |
H |
N(C4H8)2 |
Ð |
ÿ78 |
70 |
30.0(S) |
|
|
|
|
HMPA |
ÿ78 |
74 |
50.0(R) |
5 |
Ph |
Me |
N(C4H8)2 |
Ð |
ÿ78 |
77 |
60.0(R) |
|
|
|
|
HMPA |
ÿ78 |
35 |
20.0(S) |
ee ˆ Enantiomeric excess; HMPA ˆ hexamethylphosphoramide.
Reprinted with permission by Am. Chem. Soc., Ref. 111.
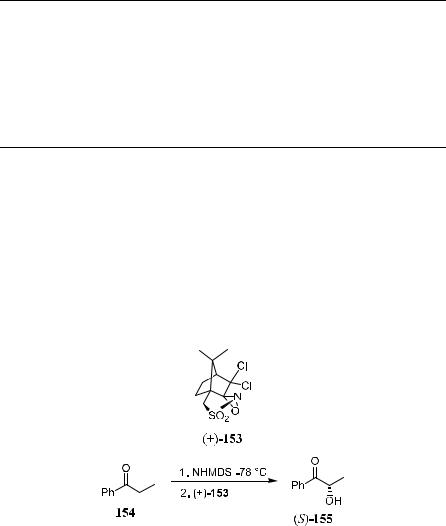
In contrast to the oxidation of prochiral esters and amides, which induces only moderate ee, sodium enolates of ketones give high stereoselectivity with (‡)-147 or (ÿ)-147 as the oxidant (Scheme 4±56 and Table 4±21). The highest stereoselectivity has been observed in the oxidation of the sodium enolate of deoxybenzoin 150, in which benzoin 149 can be obtained in over 95% optical purity.
Scheme 4±56
254 ASYMMETRIC OXIDATIONS
TABLE 4±21. Asymmetric Oxidation of Prochiral Ketone Enolates to a-Hydroxyl Ketones Using 147
Ketone |
Base |
Temp. ( C) |
Yield (%) |
ee (%) |
Con®g. |
PhCOCH2Ph |
LDA |
0 |
70 |
68 |
S |
|
NHMDS |
ÿ78 |
84 |
95.4 |
S |
PhCOCH2Me |
LDA |
0 |
51 |
43.2 |
S |
|
NHMDS |
ÿ78 |
77 |
68.5 |
S |
t-BuCOCH2Me |
LDA |
0 |
55 |
33 |
R |
|
NHMDS |
ÿ78 |
71 |
90 |
R |
PhCH2COMe |
NHMDS |
ÿ78 |
70 |
41 |
S |
|
NHMDS/HMPA |
ÿ78 |
76 |
76 |
R |
ee ˆ Enantiomeric excess; LDA ˆ lithium diisopropylamide; NHMDS ˆ NaN(SiMe3)2.
a-Hydroxy ketones, which are otherwise di½cult to prepare, can be obtained with high ee by applying the Davis reagent±mediated oxidation reaction. For example, as shown in Scheme 4±57, (S)-2-hydroxy-1-phenyl-1-propanone (S)- 155 can be generated with over 95% ee and 61% isolated yield by oxidation of the sodium enolate of 154 with (‡)-153 at ÿ78 C. Oxidation of the enolate of 154 with (‡)-147, on the other hand, yields (S)-155 in only 62% ee.112
Scheme 4±57
Following their success with chiral ketone-mediated asymmetric epoxidation of unfunctionalized ole®ns, Zhu et al.113 further extended this chemistry to prochiral enol silyl ethers or prochiral enol esters. As the resultant compounds can easily be converted to the corresponding a-hydroxyl ketones, this method may also be regarded as a kind of a-hydroxylation method for carbonyl substrates. Thus, as shown in Scheme 4±58, the asymmetric epoxidation of enol silyl

4.9 ASYMMETRIC AZIRIDINATION AND RELATED REACTIONS |
255 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±58
ether or ester gives, after subsequent treatment, the corresponding a-hydroxyl ketone in up to 99% ee.
4.9ASYMMETRIC AZIRIDINATION AND RELATED REACTIONS
Aziridines, like epoxides, have great synthetic potential and are versatile intermediates for nitrogen-containing compounds. Tanner114 reviewed di¨erent uses of optically active aziridines as chiral synthons for the enantioselective synthesis of alkaloids, amino acids, b-lactams, and pyrrolidines as well as their potential as chiral auxiliaries or chiral ligands. These aziridine compounds can be approached through reaction of nitrene with alkene, which is analogous to epoxide formation. Indeed, this has become a burgeoning area of interest in recent years.
4.9.1Asymmetric Aziridination
Various approaches to epoxide also show promise for the preparation of chiral aziridines. Identi®cation of the Cu(I) complex as the most e¨ective catalyst for this process has raised the possibility that aziridination might share fundamental mechanistic features with ole®n cyclopropanation.115 Similar to cyclopropanation, in which the generally accepted mechanism involves a discrete Cu±carbenoid intermediate, copper-catalyzed aziridation might proceed via a discrete Cu±nitrenoid intermediate as well.
Scheme 4±59
256 |
ASYMMETRIC OXIDATIONS |
|
|
||
TABLE 4±22. Asymmetric Aziridinations of Styrenes |
|
|
|||
|
|
|
|
||
Entry |
Ligand |
ee (%) |
Reference |
||
|
|
|
|
|
|
1 |
|
|
|
63 |
116 |
|
|
|
|||
|
|
|
|||
2 |
33 |
116 |
3 |
81 |
117 |
ee ˆ Enantiomeric excess.
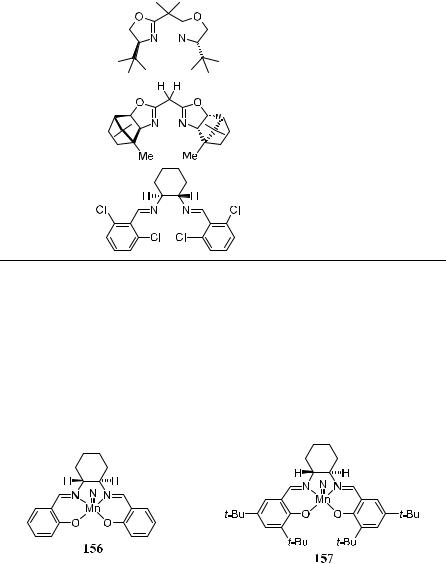
As the application of transition metal±salen complexes for asymmetric epoxidation has gained increasing recognition, chemists from many groups have also tried to use a salen complex for the asymmetric aziridination of alkenes.118 For example, the chiral nitridomanganese complexes 156 and 157 were synthesized by treating the chiral ligands with Mn(OAc)2, NH3 H2O and NaOCl or reacting Mn(III) complex with gaseous NH3 using chloramine-T as the oxidant in MeOH.
Both compounds were tested for their catalytic activity in asymmetric aziridination using p-toluenesulfonic anhydride (Ts2O) to activate the nitridomanganese complex. As shown in Scheme 4±60, the aziridination generally gave poor results, while addition of pyridine N-oxide improved both the yield and the enantiomeric excess of the products.

|
|
4.9 ASYMMETRIC AZIRIDINATION AND RELATED REACTIONS |
257 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±60. Reprinted with permission by Wiley-VCH Verlag Germany, Ref. 118.
Jeong et al.119 developed another procedure for the aziridination of ole®ns involving the inexpensive practical nitrogen source chloramine-T (TsNClNa). The reaction was not facilitated by any transition metal catalyst, but could proceed with good yield in the presence of inorganic bromides. They further found that using phenyltrimethylammonium bromide (PTAB) as the bromine source gave good to excellent yields of aziridines with a wide range of ole®n substrates. As shown in Table 4±23, aziridination of ole®ns with TsNClNa gives much higher yield than the 156 catalyzed reaction.
Although the process as it stands is still not enantioselective in nature, the high yield and mild reaction conditions may attract further search for an asymmetric version of this reaction using proper chiral bromine-comtaining compounds as the catalyst.
Porphyrin complexes, which have been mentioned in the previous section as catalysts for the epoxidation of ole®ns, can also catalyze aziridination120 using [N-( p-toluenesulfonyl)imino]phenyl iodinane or other nitrene precursors.
Asymmetric aziridination are further discussed elsewhere.121
4.9.2Regioselective Ring Opening of Aziridines
The regiospeci®c reductive ring cleavage of N-substituted aziridines has been the means of many synthetic e¨orts for the asymmetric synthesis of amino derivatives. Just like the products from the regioselective and chemoselective ring opening of chiral epoxides, these amino derivatives can be the building blocks for biologically important compounds.
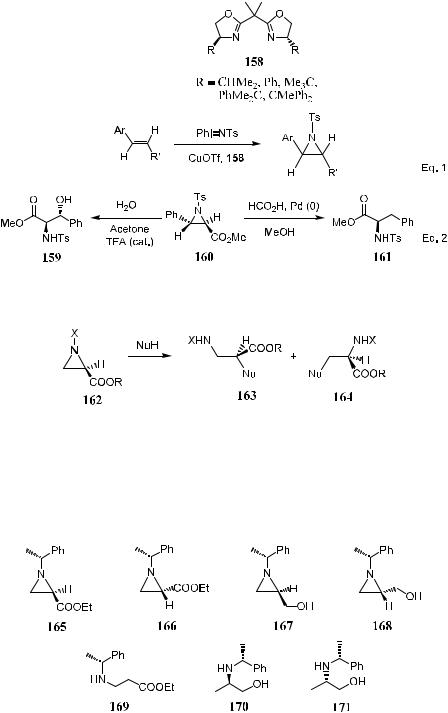
Bis(oxazoline)±copper complexes 158 have been used by Evans' group as chiral catalysts for the enantioselective aziridination of ole®ns.116 Arylsubstituted ole®ns have been found to be particularly suitable substrates, which can be e½ciently converted to N-tosylaziridines with ee of up to 97% (R ˆ Ph

258 ASYMMETRIC OXIDATIONS
TABLE 4±23. Bromide Catalyzed Aziridination of Ole®ns with TsNClNa
Entry |
Substrate |
Product |
Yield (%, isolated yield in bracket) |
1 |
|
|
93 (90) |
|
|
|
|
2 |
|
|
76 (62) |
3 |
|
|
95 (88) |
4 |
|
|
89 (72) |
5 |
|
|
86 (80) |
6 |
|
|
54 |
7 |
|
|
68 (65) |
8 |
|
|
76 (60) |
9 |
|
|
51 |
Reprinted with permission by Am. Chem. Soc., Ref. 119.
in 158 and R0 ˆ COOPh in Eq. 1, Scheme 4±61). Reductive ring opening of 160 by transfer hydrogenation a¨ords the corresponding 2-(R)-phenylalanine 161, and acidic hydrolysis of 160 yields the b-hydroxy-a-amino ester 159 (Eq. 2 in Scheme 4±61).
The regioselective reductive ring opening of N-substituted aziridine-2- carboxylates gives either a- or b-amino acids.122 Heteronucleophiles such as nitrogen,123 oxygen,124 sulfur,125 and chloride preferably attack the C-3 position to provide a-amino acid derivatives. However, a mixture of products will result when carbanion or azide is used as the nucleophile (Scheme 4±62).
Lim and Lee122 reported the regioselective reductive ring opening of N- substituted aziridine-2-carboxylates and aziridine-2-methanol via catalytic hydrogenation using Pd as a catalyst. For example, catalytic hydrogenation of 165 and 166 in AcOH with 20 mol% of Pd(OH)2 proceeded with C-2 cleavage,
4.9 ASYMMETRIC AZIRIDINATION AND RELATED REACTIONS |
259 |
Scheme 4±61
Scheme 4±62
yielding exclusively product 169. The catalytic hydrogenation of 167 and 168 in EtOH with 20 mol% of Pd(OH)2 gave the C-3 cleavage products 170 and 171 with yields over 95%.

260 ASYMMETRIC OXIDATIONS
Osborn et al.126 demonstrated the regiospeci®c ring opening of N-dimethyl phosphinoylaziridine by copper(I)-modi®ed Grignard reagents with good to excellent yields (Scheme 4±63). The process was activated by the phosphinyl group. Either primary or secondary Grignard reagents are suitable nucleophiles, and the reaction is of general utility. When R ˆ PhCH2 (172), the yields of 173 for R0 ˆ Me, n-Bu, and Ph are 67%, 89%, and 83%, respectively.
Scheme 4±63
Before closing this discussion of oxidation reactions, it is worth mentioning a demonstrated synthesis of a-aminoalkylphosphonate from vinylphosphonate via aziridinyl phosphonates, even though this reaction is thus far not asymmetric in nature.127 In the presence of a copper catalyst, vinylphosphonates of type 174 were treated with PhIbNTs, followed by the reductive ring opening of aziridinylphosphonate 175 to a¨ord a-aminoalkylphosphonate 176 in good yield (Scheme 4±64). a-Aminoalkylphosphonates are key substrates in the synthesis of phosphonopeptides.
Scheme 4±64
4.10SUMMARY
This chapter covers the asymmetric epoxidation of allylic alcohols as well as unfunctionalized ole®ns. Although Sharpless epoxidation is a useful tool for synthesizing 2,3-epoxy alcohols, it is not e¨ective for the asymmetric epoxidation of unfunctionalized ole®ns. The Jacobsen/Katsuki metal±salen com- plex-promoted reaction provides a route to unfunctionalized epoxides, but this reaction generally requires a cis-substituted substrate bearing a p-stabilizing substituent. The chiral ketone-mediated epoxidation developed by Cao et al. and Yang et al. can be considered a breakthrough in asymmetric epoxidation of unfunctionalized ole®ns, and a wide range of ole®ns can be epoxidized with high enantioselectivity using this method. As a complement to these oxidation methods, sulfur ylide±mediated carbene addition to carbonyl groups has also
