
Principles and Applications of Asymmetric Synthesis
.pdf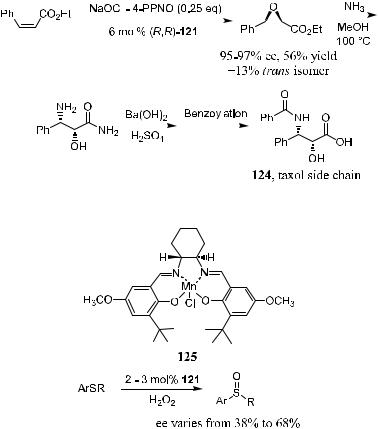
4.6 EPOXIDATION OF UNFUNCTIONALIZED OLEFINS |
241 |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±42. Synthesis of the taxol side chain.
Scheme 4±43. Extension of Salen-complex to the Oxidation of sul®des.
or large-scale reactions due to their instability in the solid state, their lack of solubility in organic solvents, their relatively high cost, and the high molecular weight of the oxygen transfer by-product. Compared with iodosylbenzene, hydrogen peroxide gives higher yields of sulfoxide, minimal overoxidation to sulfone, and enantioselectivities identical to those observed with iodosylbenzene.
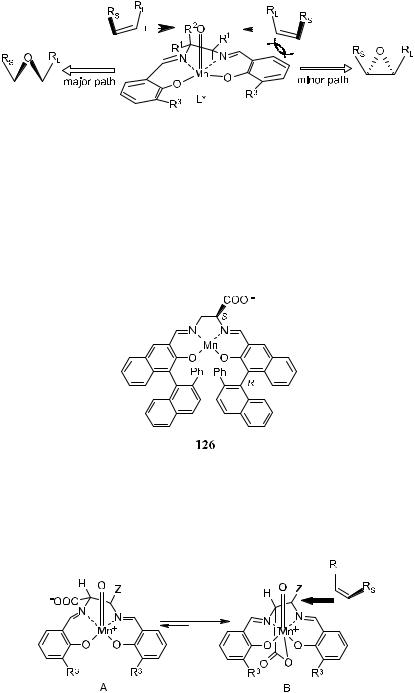
Many e¨orts have been made to develop salen catalysts for the epoxidation of unfunctionalized ole®ns, and such work has been well documented.93 Very recently, Ito and Katsuki94 proposed that the ligand of the oxo salen species is not planar, but folded as shown in Figure 4±7 (R0 0 H, R2 ˆ H, L ˆ achiral axial ligand). This folded chiral structure ampli®es asymmetric induction by the Mn±salen complex. This transition state proposed by Ito and Katsuki is not compatible with the proposal by Palucki et al.95 that the salen ligands of oxo species are planar.
The conformation bearing the substituent at the asymmetric center in a pseudoequatorial position (R1) is more stable than that bearing the substituent
242 ASYMMETRIC OXIDATIONS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S ˆ |
|
ˆ |
aryl; R1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Figure 4±7. |
Folded |
conformation of the salen complex. R |
Alkyl; RL |
, |
||||||||||||||
R |
2 |
|
|
|
aryl; R |
3 |
|
|
|
|
|
|
|
|
|
|||
|
ˆ H, alkyl, or |
|
ˆ bulky group. Reprinted with permission |
by Elsevier |
||||||||||||||
Science Ltd., Ref. 94.
in a pseudoaxial position (R2). It is this conformation that controls the asymmetric induction of the reaction. Based on the proposed nonplanarity of the salen ligand, Katsuki's group prepared a conformationally reversed optically active salen±manganese(III) complex 126 bearing a carboxylate group on the ethylenediamine moiety.94
Reversal of the conformation of the chiral Mn±salen complex forces the substituents on the ethylenediamine moiety to take the disfavored axial position. This disfavored conformation (Fig. 4±8A) should be stabilized by the co-
Figure 4±8. Transition state for 126 catalyzed epoxidation.
4.6 EPOXIDATION OF UNFUNCTIONALIZED OLEFINS |
243 |
ordination of carboxylate to the Mn ion (Fig. 4±8B). Complex 126 was then found to be an e½cient catalyst for the asymmetric epoxidation of several 2,2- dimethylchromene derivatives, resulting in high ee (up to 99%) and high turnover number (up to 9200).
4.6.2 Catalytic Enantioselective Epoxidation of Simple Ole®ns by Porphyrin Complexes
Porphyrin±metal complexes are natural mimetic substances that have attracted much attention during the past decade. The epoxidation of ole®ns by porphyrin complexes proceeds well, but with only modest enantioselectivity. As this area of research is growing, description of a few selected publications may be useful.96
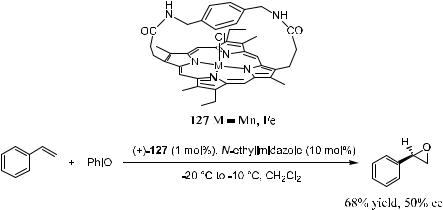
Konishi et al.97 synthesized porphyrin compound 127. As shown in Scheme 4±44, asymmetric epoxidation of prochiral ole®ns such as styrene derivatives and vinyl naphthalene by iodosobenzene has been achieved by using this porphyrin complex as the catalyst in the presence of imidazole. The optically active epoxides were obtained with moderate ee.
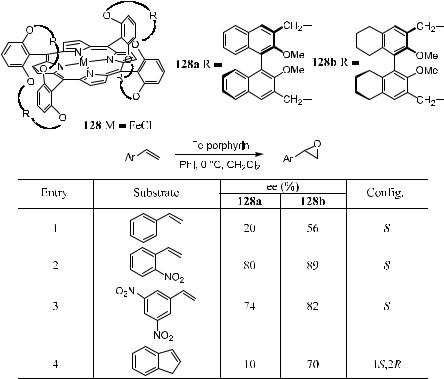
Better results for the porphyrin complex±catalyzed asymmetric epoxidation of prochiral ole®ns were achieved by Naruta et al.98 using iron complexes of chiral binaphthalene or bitetralin-linked porphyrin 128 as chiral catalysts. As shown in Scheme 4±45, asymmetric epoxidation of styrene or its analogs provided the product with good ee. Even better results were obtained with substrates bearing electron-withdrawing substituents.
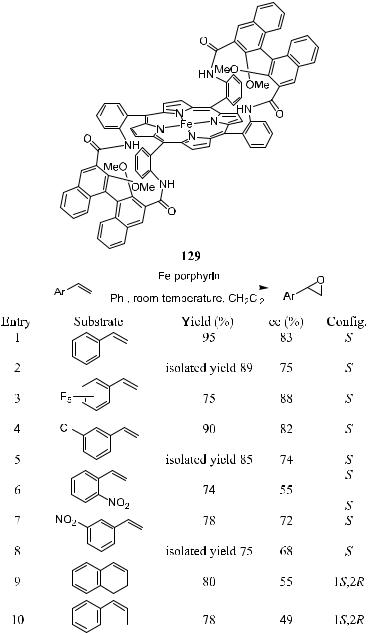
Collman et al.99 reported the asymmetric epoxidation of terminal ole®ns catalyzed by iron porphyrin complex 129. The catalyst was synthesized by connecting binaphthyl moieties to a readily available aabb-tetrakis(aminophenyl)- porphyrin (TAPP). Epoxidation of unfunctinalized ole®ns was carried out using iodosylbenzene as the oxidant. As shown in Scheme 4±46, excellent results were
Scheme 4±44
244 ASYMMETRIC OXIDATIONS
Scheme 4±45
obtained for most substrates. The good ee values, high turnover number, and ready availability of the reagents make this compound a potential catalyst for many practical applications.
4.6.3 Chiral Ketone±Catalyzed Asymmetric Oxidation of
Unfunctionalized Ole®ns
As oxiranes can be generated in situ from Oxone9 (potassium peroxomonosulfate) and a ketone, dioxiranes are attractive oxidants for epoxidation reactions that may be rapid and may require only a simple workup.
The following rules should be considered when designing this type of chiral ketone compound: (1) The stereogenic centers should be close to the reaction centers in order to get e½cient stereochemical communication between the substrate and the catalyst; (2) the presence of a fused ring and a quaternary center a to the carbonyl group will minimize the epimerization of the stereogenic center; and (3) one face of the catalyst should be sterically blocked in order to limit possible competing approaches from this face. In addition, it is essential to design the catalyst bearing a C2-symmetric axis.
|
|
|
|
|
4.6 EPOXIDATION OF UNFUNCTIONALIZED OLEFINS |
245 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±46. Reprinted with permission by Am. Chem. Soc., Ref. 99.
246 ASYMMETRIC OXIDATIONS
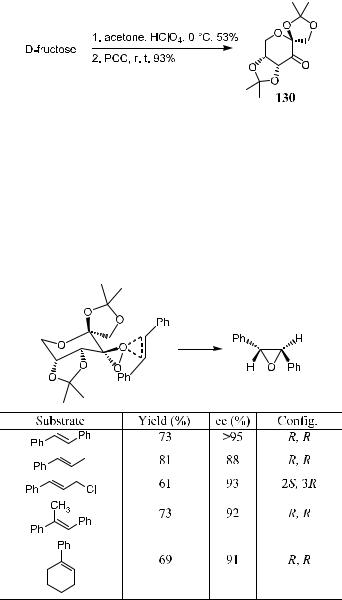
4.6.3.1 Chiral Ketone from Carbohydrate. Tu et al.100 reported a dioxir- ane-mediated asymmetric epoxidation based on the ketones derived from the low cost material d-fructose (Scheme 4±47).
Scheme 4±47
All the reactions were carried out at 0 C, with the substrate (1 equivalent), ketone (3 equivalents), Oxone9 (5 equivalents), and NaHCO3 in CH3CN± aqueous EDTA for 2 hours. High enantioselectivity can generally be obtained for transand trisubstituted ole®ns. The favored spiro and planar transition states have been proposed for ketone 130-mediated trans-stilbene epoxidation (Scheme 4±48).
Scheme 4±48. Reprinted with permission by Am. Chem. Soc., Ref. 100.
4.6 EPOXIDATION OF UNFUNCTIONALIZED OLEFINS |
247 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4±49
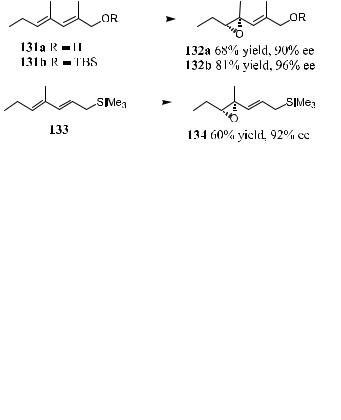
In most cases, such reactions proceed highly regioselectively.101 Taking the epoxidation of dienol 131a, 131b, and 133 as an example, as shown in Scheme 4±49, under such conditions the nonallylic double bonds are epoxidized, giving the corresponding monoepoxides 132a, 132b, and 134, respectively. Only trace amounts of the bis-epoxides are detected in the crude products.
Cao et al.102 extended their discovery to the asymmetric epoxidation of enynes using ketone 130 as the catalyst and Oxone9 as the oxidant (Scheme 4±50).
Scheme 4±50
Subsequently, high chemoselectivity and enantioselectivity have been observed in the asymmetric epoxidation of a variety of conjugated enynes using fructose-derived chiral ketone as the catalyst and Oxone9 as the oxidant. Reported enantioselectivities range from 89% to 97%, and epoxidation occurs chemoselectively at the ole®ns. In contrast to certain isolated trisubstituted ole®ns, high enantioselectivity for trisubstituted enynes is noticeable. This may indicate that the alkyne group is bene®cial for these substrates due to both electronic and steric e¨ects.
Mechanistic studies103 revealed that chiral ketone-mediated asymmetric epoxidation of hydroxyl alkenes is highly pH dependent. Lower enantioselectivity is obtained at lower pH values; at high pH, epoxidation mediated by chiral ketone out-competes the racemic epoxidation, leading to higher enantioselectivity. (For another mechanistic study on ketone-mediated epoxidation of CbC bonds, see Miaskiewicz and Smith.104)
248 ASYMMETRIC OXIDATIONS
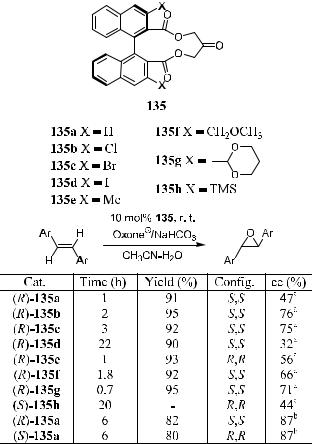
4.6.3.2 A C2 Symmetric Chiral Ketone for Catalytic Asymmetric Epoxidation of Unfunctionalized Ole®ns. Yang et al.105 reported the use of C2-symmetric chiral ketones 135a±h for the asymmetric epoxidation of unfunctionalized ole®ns using Oxone9 as the oxidant. Moderate to good enantioselectivities were obtained for trans-ole®ns and trisubstituted ole®ns (33±87% ee) (Scheme 4±51). X-ray structural analysis of 135a shows that this chiral ketone has a rigid structure and C2 symmetry. The keto group lies on the C2 axis of the molecule, and the two ester groups are nearly perpendicular to the plane of the macrocyclic ring. The two naphthalenes are located on the opposite face of the ketone group, and the dihedral angle of the two naphthalene rings is about 70 .
Scheme 4±51. Asymmetric epoxidation of trans-stilbene with catalyst 135. aSubstrate: (E )-stilbene. bSubstrate: (E )-4,40-diphenylstilbene. Reprinted with permission by Am. Chem. Soc., Ref. 105a, 106.
4.7 CATALYTIC ASYMMETRIC EPOXIDATION OF ALDEHYDES |
249 |
Yang et al.106 found that when the para substituents of the trans-stilbenes became larger, the ee values of the trans-epoxides increased gradually. Their studies show that these chiral ketones are stable under the reaction conditions and can be recovered in over 80% yield and reused without loss of catalytic activity and chiral induction. The ester groups that give the rigid and C2- symmetric structure to the cyclic ketones seem to be essential for e¨ective asymmetric epoxidation. Lowering the reaction temperature enhances the enantioselectivity but decreases the reaction rate. The most suitable reaction temperature is 0 C, at which optimal yields and ee values can be obtained.
4.7CATALYTIC ASYMMETRIC EPOXIDATION OF ALDEHYDES
Thus far, the asymmetric epoxidation of ole®ns for preparing chiral epoxide compounds has been discussed. These are invaluable intermediates in the organic synthesis of important molecules such as pharmaceuticals or agrochemicals. The Sharpless and Jacobsen/Katsuki methods have proved to be the most powerful for preparing chiral epoxides of various types. Some drawbacks to these reactions may be that Sharpless epoxidation requires an allylic alcohol and the Jacobsen/Katsuki epoxidation generally requires a cis-substituted substrate bearing a p-stabilizing substituent. The chiral ketone-mediated epoxidation developed by Cao and Yang can be considered a breakthrough in asymmetric epoxidation of unfunctionalized ole®ns, and a wide range of ole®ns can now be epoxidized with high enantioselectivity.
An alternative to the synthesis of epoxides is the reaction of sulfur ylide with aldehydes and ketones.107 This is a carbon±carbon bond formation reaction and may o¨er a method complementary to the oxidative processes described thus far. The formation of sulfur ylide involves a chiral sul®de and a carbene or carbenoid, and the general reaction procedure for epoxidation of aldehydes may involve the application of a sul®de, an aldehyde, or a carbene precursor as well as a copper salt. This reaction may also be considered as a thiol acetalmediated carbene addition to carbonyl groups in the aldehyde.
In the design of chiral sul®des for sulfur ylide±mediated asymmetric epoxidation of aldehydes, two factors are important. First, a single sulfur ylide should be produced. Otherwise, the diastereomeric sulfur ylides may react with aldehydes in di¨erent ways and thus cause a drop in stereoselectivity. This may be achieved by choosing a rigid cyclic structure to make one of the lone pairs more accessible than the other. Second, the structure should be amenable to structural modi®cation in order to study the electronic and steric e¨ects of the sulfur on the enantioselectivity of the epoxidation reaction.
Aggarwal et al.108 reported excellent results with the catalytic asymmetric epoxidation of aldehydes. As shown in Scheme 4±52, a series of thioacetals 137 was prepared from hydroxy thiol 136 and the corresponding carbonyl compound. Among them, compound 138, derived from 136 and acetaldehyde, proved to be the best catalyst for asymmetric epoxidation of aldehydes.

250 ASYMMETRIC OXIDATIONS
Scheme 4±52
This reaction is very sensitive to water because in the presence of water and a metal salt (such as copper salt) the thioacetal tends to decompose, and this may reduce the amount of thioacetal available for epoxidation. When water is excluded from all the reagents, the reaction can be carried out in the presence of a catalytic amount of thioacetal. Otherwise, a stoichiometric amount of thioacetal compound is required. Scheme 4±53 summarizes the epoxidation of aldehydes using 138 as the chiral-inducing reagent. Excellent enantioselectivities are obtained in most cases.
4.8 ASYMMETRIC OXIDATION OF ENOLATES FOR THE PREPARATION OF OPTICALLY ACTIVE a-HYDROXYL CARBONYL COMPOUNDS
In a general sense, oxidation refers to the introduction of oxygen or other electronegative atoms to organic molecules. In this context, a-hydroxylation of a
Scheme 4±53
