
Wasserscheid P., Welton T. - Ionic Liquids in Synthesis (2002)(en)
.pdf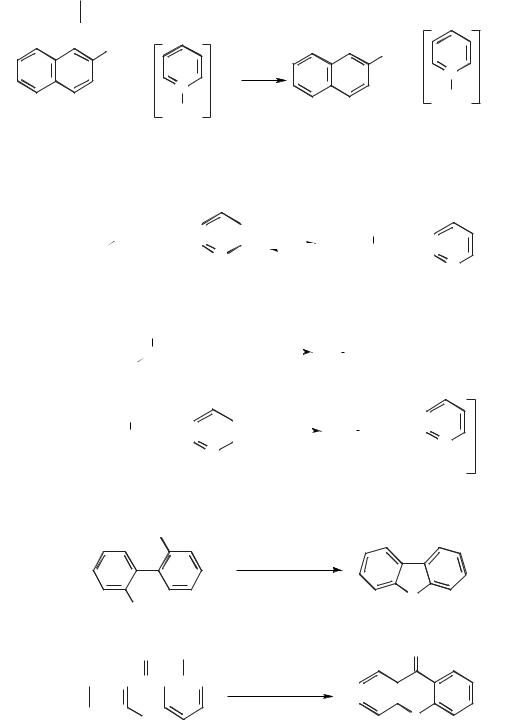
176 Martyn Earle
OCH3 |
|
|
OH |
+ |
+ |
+ |
Cl- |
+ |
N Cl- |
N |
|
|||
|
|
|
CH3 |
|
|
H |
|
|
|
|
|
|
|
Scheme 5.1-2: The demethylation of 2-methoxynaphthalene to 2-naphthol with pyridinium
chloride.
|
|
O |
+ |
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
+ |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O+ |
|
|
|
|
|
|
|
||||||||||
A r |
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
A r |
R |
|
|
|
|
|
|
N |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
H |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
+ C l- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
A r |
O+ |
|
|
|
|
|
|
|
|
|
A r OH + R |
|
|
C l |
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A r OH |
+ |
|
|
|
|
|
|
|
|||
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
O+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
A r |
|
|
R |
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
Scheme 5.1-3: A mechanism for the dealkylation of aryl ethers with pyridinium chloride.
C H3O
[PyH]Cl
O
OCH3
O OCH3 O 
 [PyH]Cl
[PyH]Cl 


 SCH3 S
SCH3 S
Scheme 5.1-4: Two examples of aryl demethylation reactions followed by cyclization.

5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 177
Tetrabutylammonium fluoride (TBAF) is usually used in the form of the trihydrate or as a solution in tetrahydrofuran (THF). The pure form is difficult to isolate, owing to decomposition to HF, tributylamine, and but-1-ene [18, 19] on dehydration. It has been used for a variety of reactions, including as a catalyst for various reactions with silicon compounds [20, 21]. One of its main uses is in the cleavage of silyl ether protecting groups [22].
TBAF has been used as a source of fluoride ions in a number of substitution reactions studied by Cox et al. [23]. Alkyl and acyl halides react with TBAF to give the corresponding alkyl or acyl fluoride in good yield. In the reaction between (R)-2- tosyloctane and TBAF, the product was (S)-2-fluorooctane, confirming an SN2 mechanism for the reaction (Scheme 5.1-5) [18, 23].
TBAF has also been used in the preparation of various fluorocarbenes. This involved the photolysis of phenylor phenoxyfluorodiazirine, which was in turn synthesized from the reaction between TBAF and phenyl or phenoxy halodiazirine, as shown in Scheme 5.1-6 [24, 25].
5.1.1.2Reactions in chloroaluminate(III) and related ionic liquids*
Reactions in chloroaluminate(III) salts and other related binary salts often proceed smoothly to give products. However, it should be noted that these salts are watersensitive and must be handled under dry conditions. They react with water to give hydrated aluminium(III) ionic species and HCl. When a reactant or product contains a heteroatomic functional group, such as a ketone, a strong ketone/aluminium(III) chloride adduct is formed. In these cases, this adduct can be difficult to separate from the ionic liquid at the end of a reaction. The isolation of the product often
[Bu4 N] F + |
H3C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C H3 |
|
|
|
|
||||
H |
|
|
|
OSO2PhCH3 |
|
|
F |
|
|
H |
+ |
[Bu4N][OSO2PhCH3] |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
H3C (C H2)5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(CH2)5CH3 |
|
|
|
||||
Scheme 5.1-5: The use of TBAF in an SN2 reaction. |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
X |
|
Ar |
[Bu4N]F |
F |
|
Ar |
hv |
|
|
.. |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
C |
||||||||||||
N |
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ar |
F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
via |
|
|
Ar |
|
X |
N |
|
|
N |
|
|||||||||||||
Ar = Ph, OPh |
|
N |
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
X = Cl, Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Scheme 5.1-6: The use of TBAF in the preparation of a fluorodiazirine.
*Chloroaluminate(III) salts are described in more detail in Chapter 2. The composition of a tetrachloroaluminate(III) ionic liquid is best described in this chapter by the apparent
mole fraction of AlCl3 {X(AlCl3)} present.
Ionic liquids with X(AlCl3) < 0.5 contain an excess of Cl– ions over [Al2Cl7]– ions, and are
termed “basic”; those with X(AlCl3) > 0.5 contain an excess of [Al2Cl7]– ions over Cl–, and are termed “acidic”; melts with X(AlCl3) = 0.5 are termed 'neutral'. For example, the binary salt NaCl/AlCl3 (X(AlCl3) = 0.67) refers to a 1 part NaCl to 2 parts AlCl3 mixture of salts and is described as “acidic”.
178 Martyn Earle
NaCl-AlCl3 (X = 0.69)
270 °C / 4 min.
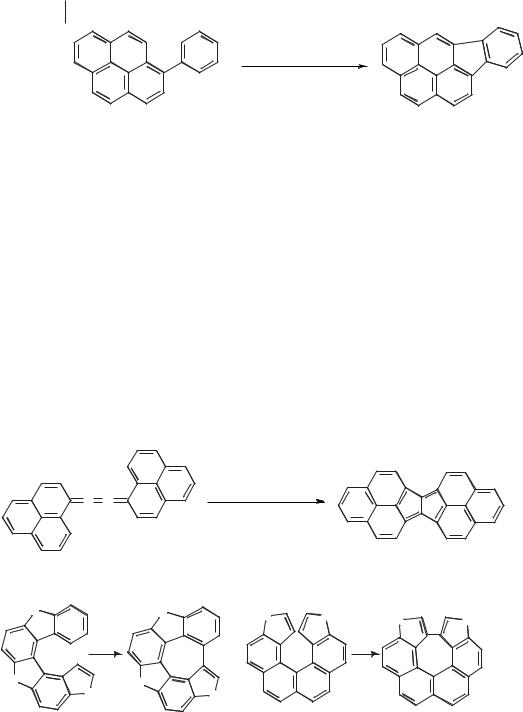
Scheme 5.1-7: The Scholl reaction of 1-phenylpyrene.
involves destruction of the ionic liquid with water. For products that do not have polar electron-donating functional groups, isolation of the products is straightforward and the ionic liquid can be reused.
One of the first reactions to be carried out in a molten salt (albeit at 270 °C) was the Scholl reaction. This involves the interor intramolecular coupling of two aromatic rings. A example of this reaction, in which 1-phenylpyrene was cyclized to indeno[1,2,3-cd]pyrene [26] is given in Scheme 5.1-7. A more elaborate version of the Scholl reaction is shown in Scheme 5.1-8 and involves bicyclization of an aromatic cumulene [27].
Wynberg et al. found that the yields in the cyclization of helicines could be improved from 10 % in an aluminium(III) chloride solution in benzene system to 95 % in a NaCl/AlCl3 (X(AlCl3) = 0.69) molten salt [28]. An example is given in Scheme 5.1-9.
The Scholl reaction involves an overall oxidation of the coupled aromatic rings, yet there is no obvious oxidizing agent. This poses the question of what happens to the two hydrogen atoms that are produced in this reaction. It has been suggested that oxygen (air) may act as the oxidant, but this currently lacks confirmation [18].
C C |
|
NaCl-AlCl3 (X = 0.69) |
|
|
|
|
230 °C |
|
|
|
|
|
|
|
|
|
|
Scheme 5.1-8: The cyclisation of an aromatic cumulene in a molten salt. |
|
|
|||
S |
S |
S |
S |
S |
S |
|
|
||||
|
|
|
|
||
a |
|
|
a |
|
|
95 % |
|
|
93 % |
|
|
S |
S |
|
|
|
|
S |
|
S |
|
|
|
Scheme 5.1-9: The Scholl reactions of two helicines. (a = NaCl/AlCl3 (X(AlCl3) = 0.69) at 140 °C).
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 179
N H2  N H2
N H2
2
NaCl-KCl-AlCl3
200 °C
H2N
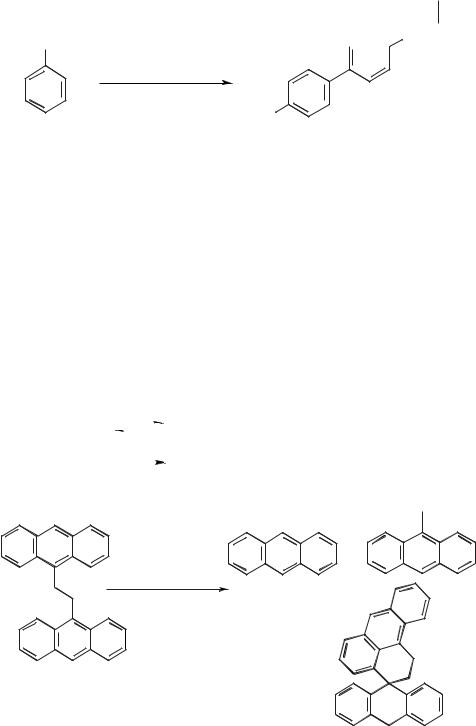
Scheme 5.1-10: The dimerization of aniline to benzidine in a molten salt.
The molten salt NaCl/KCl/AlCl3 (20:20:60) was used in the dimerization of aniline to form benzidine (Scheme 5.1-10) [29].
Buchanan and co-workers studied the behavior of various aromatic compounds in antimony(III) molten salts [30]. These salts can act both as mild Lewis acids and allow redox reactions to take place. The Lewis acidity of the melt can be tuned by controlling the concentration of [SbCl2]+. Basic melts are formed by addition of a few mol % of a chloride donor such as KCl, whereas acidic melts are formed by addition of chloride acceptors such as AlCl3 (Scheme 5.1-11).
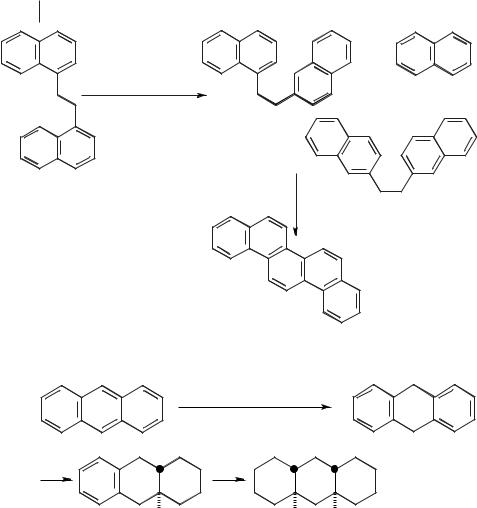
Examples of reactions that have been carried out in these antimony(III) ionic liquids include the cyclizations of 1,2-bis-(9-anthryl)-ethane (Scheme 5.1-12) and 1,2- bis-(1-naphthyl)-ethane (Scheme 5.1-13). A more detailed review of antimony(III) chloride molten salt chemistry has been published by Pagni [4].
Polycyclic aromatic hydrocarbons dissolve in chloroaluminate(III) ionic liquids to give brightly colored solutions (due to the protonated aromatic compound [31]). The
SbCl3 |
|
|
|
[SbCl2]+ |
+ |
Cl- |
|
|
|
|
|||||
|
|
|
|||||
SbCl3 + AlCl3 |
|
|
|
[SbCl2]+ |
+ |
[AlCl4]- |
acidic |
|
|
|
Scheme 5.1-11: The effect of the addition of aluminium(III) chloride to antimony(III) chloride.
+
SbCl3-KCl (X = 0.1)
80 °C, 30 min
+
Scheme 5.1-12: The cyclisation of 1,2-bis-(9-anthryl)-ethane in antimony(III) ionic liquids.
180 Martyn Earle
+
SbCl3-AlCl3 (X = 0.1)
80 °C, 2 min
+
longer reaction time
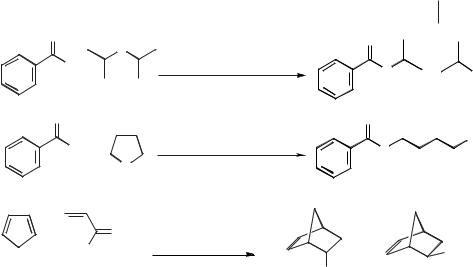
Scheme 5.1-13: Reactions of bisnaphthylethane in antimony(III) ionic liquids.
[ EMIM]Cl-AlCl3 (X= 0.67) Zn / HCl
H |
H H |
Yield = 90 % |
as a single isomer |
Scheme 5.1-14: The reduction of anthracene to perhydroanthracene.
addition of a reducing agent (such as an electropositive metal and a proton source) results in the selective hydrogenation of the aromatic compound. For example, pyrene and anthracene can be reduced to perhydropyrene and perhydroanthracene at ambient temperatures and pressures (Scheme 5.1-14). Interestingly, only the thermodynamically most stable isomer of the product is obtained [32]. This contrasts with catalytic hydrogenation reactions, which require high temperatures and pressures and expensive platinum oxide catalysts and give rise to isomeric mixtures of products.
Singer and co-workers have shown that benzoyl chloride reacts with ethers to give alkyl benzoates [33] in chloroaluminate(III) ionic liquids. This reaction results in
|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 181 |
|
|||
O |
|
|
O |
|
|
|
|
O |
|
||
|
|
+ |
|
||
Cl |
|
[EMIM]I-AlCl3 (X=0.67) |
|
||
+ |
O |
I |
|||
|
|
||||
|
|
|
|
||
O |
|
|
O |
|
|
Cl + |
[EMIM]I-AlCl3 (X =0.67) |
O |
I |
||
|
|||||
|
|
O |
|
|
|
Scheme 5.1-15: The acylative cleavage of ethers in an ionic liquid.
+ |
O |
[EMIM]Cl-AlCl3 |
|
|
(X = 0.48 or 0.51) |
+ |
|||
|
CH3O |
|||
|
endo |
CO2CH3 |
||
|
|
exo |
||
|
|
|
CO2CH3 |
Scheme 5.1-16: The Diels-Alder reaction in a chloroaluminate(III) ionic liquid.
the acylative cleavage of ethers, and a number of reactions with cyclic and acyclic ethers have been investigated in the ionic liquid [EMIM]I/AlCl3 (X(AlCl3) = 0.67). Two examples are shown in Scheme 5.1-15.
Esterification reactions can be catalyzed by the ionic liquid 1-butylpyridinium chloride-aluminium chloride ([BP]Cl/AlCl3 (X(AlCl3) = 0.33) [34, 35]. Deng and coworkers found that higher yields were obtained than in similar reactions with a sulfuric acid catalyst.
Lee has used chloroaluminate(III) ionic liquids in the Diels–Alder reaction [36]. The endo:exo ratio rose from 5.25 to 19 on changing the composition of the ionic liquid from X(AlCl3) = 0.48 to X(AlCl3) = 0.51 (Scheme 5.1-16). The reaction works well, giving up to 95 % yield, but the moisture-sensitivity of these systems is a major disadvantage, the products being recovered by quenching the ionic liquid in water.
5.1.1.3Reactions in neutral ionic liquids
Chloroaluminate(III) ionic liquids are excellent media in many processes, but suffer from several disadvantages, such as their moisture-sensitivity and the difficulties in separation of products containing heteroatoms. Furthermore, these ionic liquids often have to be quenched (usually in water) at the end of a chemical reaction, and are lost in the form of acidic aqueous waste. Research is, therefore, shifting to the investigation of ionic liquids that are more stable to water. This allows for straightforward product separation and ease of handling. In particular, a number of ionic liquids have been found to be hydrophobic (immiscible with water), but readily dissolve many organic molecules (with the exception of alkanes, some ethers, and alkylated aromatic compounds such as toluene). An example of this is the ionic liquid [BMIM][PF6] [37], which forms triphasic solutions with alkanes and water [38]. This multiphasic behavior has important implications for clean synthesis and is analogous to the use of fluorous phases in some chemical processes [39]. For
182 Martyn Earle
example, a reaction can be performed in the ionic liquid, the products separated by distillation or steam stripping, and a by-product extracted with water or an organic solvent.
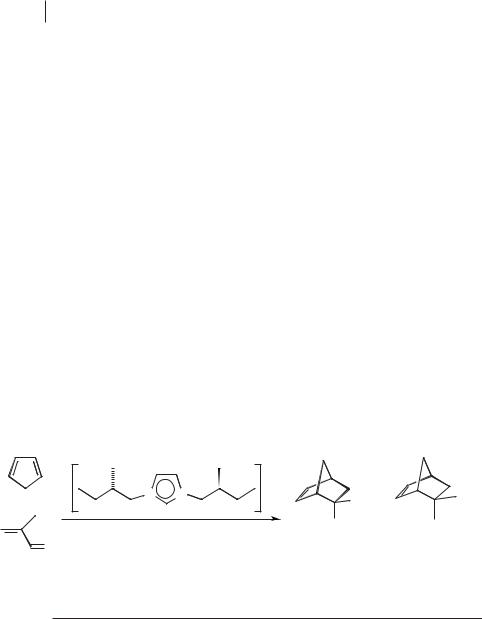
Diels–Alder reactions Neutral ionic liquids have been found to be excellent solvents for the Diels–Alder reaction. The first example of a Diels–Alder reaction in an ionic liquid was the reaction of methyl acrylate with cyclopentadiene in [EtNH3][NO3] [40], in which significant rate enhancement was observed. Howarth et al. investigated the role of chiral imidazolium chloride and trifluoroacetate salts (dissolved in dichloromethane) in the Diels–Alder reactions between cyclopentadiene and either crotonaldehyde or methacroline [41]. It should be noted that this paper describes one of the first examples of a chiral cationic ionic liquid being used in synthesis (Scheme 5.1-17). The enantioselectivity was found to be < 5 % in this reaction for both the endo (10 %) and the exo (90 %) isomers.
A study of the Diels–Alder reaction was carried out by Earle et al. [42]. The rates and selectivities of reactions between ethyl acrylate (EA) and cyclopentadiene (CP) in water, 5 M lithium perchlorate in diethyl ether (5 M LPDE), and [BMIM][PF6] were compared. The reactions in the ionic liquid [BMIM][PF6] were marginally faster than in water, but both were slower than in 5 M LPDE [42, 43] (see Table 5.1-1 and Scheme 5.1-18). It should be noted that these three reactions give up to 98 % yields if left for 24 hours. The endo:exo selectivity in [BMIM][PF6] was similar to that in 5 M LPDE, and considerably greater than that in water (Table 5.1-1).
In the reaction between isoprene (IP) and methyl vinyl ketone (MVK), the selectivities between the two isomers produced in this reaction can be improved from 4:1 to 20:1 by the addition of a mild Lewis acid such as zinc(II) iodide (5 mol %) to the ionic liquid [BMIM][PF6] (Scheme 5.1-18). One of the key benefits of this is that the
|
N + |
N |
Br- |
|
CHO |
|
+ |
CH3 |
+ |
||||
|
|
|||||
dichloromethane, 48 hours, -25°C |
CHO |
|
CH3 |
|||
|
|
|||||
O |
|
|
endo |
|
exo |
|
Scheme 5.1-17: |
Use of a chiral ionic liquid in a Diels-Alder reaction. |
|
|
|||
Table 5.1-1: Diels-Alder reactions in various solvents.
Solvent |
Diene |
Dienophile |
Product |
Time |
Yield |
a:b ratio |
|
|
|
|
|
|
|
[BMIM][PF6] |
CP |
EA |
1a + 1b |
1 |
36 |
8.0 |
5 M LPDE |
CP |
EA |
1a + 1b |
1 |
61 |
8.0 |
Water |
CP |
EA |
1a + 1b |
1 |
30 |
3.5 |
[BMIM][PF6]a |
IP |
MVK |
2a + 2b |
6 |
98 |
20 |
[BMIM][PF6] |
IP |
MVK |
2a + 2b |
18 |
11 |
4 |
a 5 mol % ZnI2 added, IP = isoprene.
|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids |
183 |
||
|
O |
|
1a |
|
|
solvent |
|
1b |
|
+ |
|
+ |
|
|
|
OC2H5 |
|
CO2C2H5 |
|
CP |
EA |
|
||
endo |
exo |
|
||
|
|
|
||
|
|
|
CO2C2H5 |
|
|
O |
|
|
|
+ |
[BMIM][PF6] |
|
2a |
2b |
|
|
|
+ |
|
IP |
MVK |
|
COCH3 |
COCH3 |
Scheme 5.1-18: The Diels-Alder reaction in different solvents (results are given in Table 5.1-1).
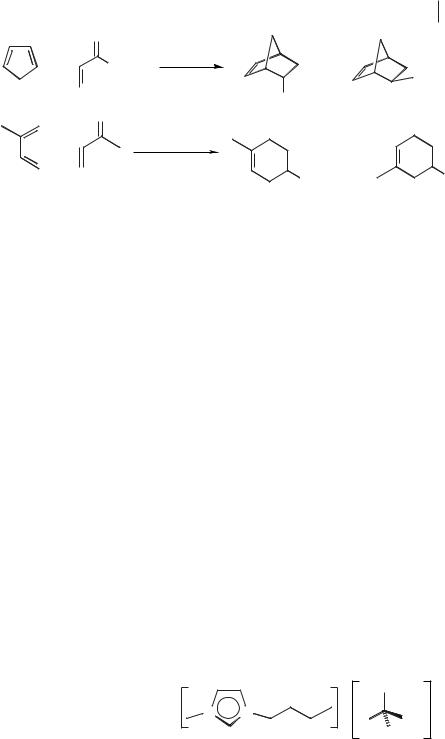
ionic liquid and catalyst can be recycled and reused after solvent extraction or direct distillation of the product from the ionic liquid. The reaction was also carried out in the chiral ionic liquid [BMIM][lactate] (Figure 5.1-1). This was found to give the fastest reaction rates of all the ionic liquids tested, and also the lowest endo:exo selectivity. The products of the Diels–Alder reaction were found to be racemic and no chiral induction was observed [42].
A similar study performed by Welton and co-workers studied the rate and selectivities of the Diels–Alder reaction between cyclopentadiene and methyl acrylate in a number of neutral ionic liquids [44]. It was found that endo:exo ratios decreased slightly as the reaction proceeded, and were dependent on reagent concentration and ionic liquid type. Subsequently, they went on to demonstrate that the ionic liquids controlled the endo:exo ratios through a hydrogen bond (Lewis acid) interaction with the electron-withdrawing group of the dienophile.
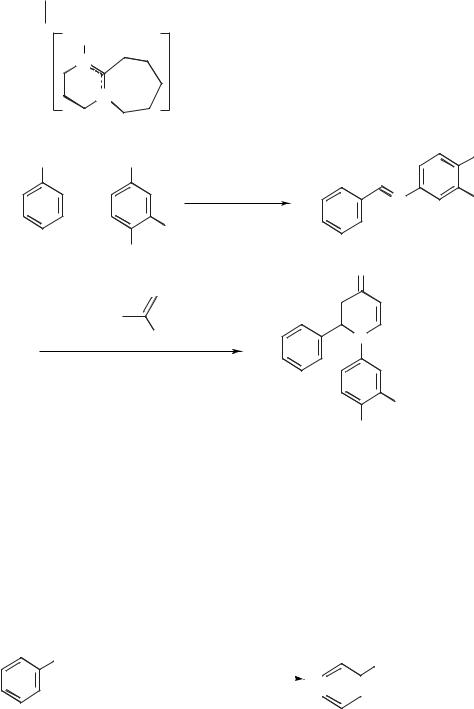
The use of molten salts based on phosphonium tosylates has also been reported for Diels–Alder reactions [45]. These salts have higher melting points than most ionic liquids in common use, and so the reactions were performed in a sealed tube. The authors claim very high selectivities in the reactions between isoprene and MVK or methyl acrylate. A new class of room-temperature ionic liquids based on phosphonium salts has been described, and has also been used for a number of Diels–Alder reactions [5]. Kitazume and Zulfiqar have investigated the aza- Diels–Alder reaction in 1-ethyl-1,8-diazabicyclo[5,4,0]undec-7-enium trifluoromethanesulfonate [EDBU][OTf] [46] (Figure 5.1-2). This reaction involved the scandium(III) trifluoromethanesulfonate-catalyzed reaction between an imine (usually generated in situ from an aldehyde and an amine) and a diene. An example of this reaction is given in Scheme 5.1-19. The yields in this reaction were high (80–99 %) and it was found that the ionic liquid could be recycled and reused.
|
CO |
- |
|
2 |
|
Figure 5.1-1: An example of a chiral |
N + N |
OH |
ionic liquid used in the Diels–Alder |
H |
|
reaction. |
CH3 |
|
184 Martyn Earle
C2H5 |
Figure 5.1-2: The structure of 1-ethyl-1,8-diazabicy- |
|
clo[5,4,0]undec-7-enium trifluoromethanesulfonate |
|
|
N |
|
|
[EDBU][OTf]. |
|
|
+ |
[OTf]- |
|
N |
|
|
CHO |
NH2 |
F |
|
||
+ |
N |
F |
[EDBU][OTf]
F
F
O
H3CO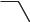
OSi(CH3)3 |
N |
Sc(OTf)3 / [EtDBU][OTf]
F
F
Scheme 5.1-19: The aza-Diels-Alder reaction in an ionic liquid.
Nucleophilic displacement reactions One of the most common reactions in organic synthesis is the nucleophilic displacement reaction. The first attempt at a nucleophilic substitution reaction in a molten salt was carried out by Ford and co-work- ers [47, 48, 49]. Here, the rates of reaction between halide ion (in the form of its triethylammonium salt) and methyl tosylate in the molten salt triethylhexylammonium triethylhexylborate were studied (Scheme 5.1-20) and compared with similar reactions in dimethylformamide (DMF) and methanol. The reaction rates in the molten salt appeared to be intermediate in rate between methanol and DMF (a dipolar aprotic solvent known to accelerate SN2 substitution reactions).
O |
|
|
|
|
|
|
|
|
O |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
- |
|
||||||
|
S |
|
OCH3 |
|
[Et3NC6H13][Et3BC6H13] |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
+ X- |
|
|
|
|
|
S O |
+ H3C-X |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
O |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5.1-20: The reaction between halide and methyl tosylate in triethylhexylammonium triethylhexylborate.
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 185
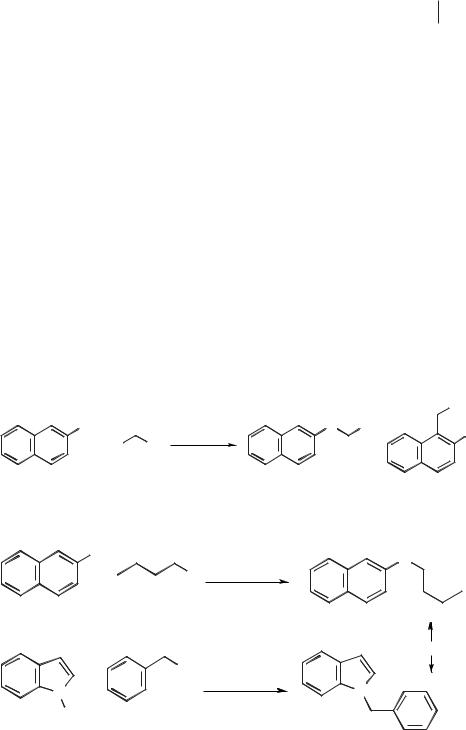
The alkylation of sodium 2-naphthoxide with benzyl bromide in tetrabutylammonium and tetrabutylphosphonium halide salts was investigated by Brunet and Badri [50] (Scheme 5.1-21). The yields in this reaction were quantitative, and alkylation occurred predominantly on the oxygen atom of the naphthoxide ion (typically 93–97 %). The rate of the reaction was slower in the chloride salts, due to the benzyl bromide reacting with chloride ion to give the less reactive benzyl chloride.
Indole and 2-naphthol undergo alkylation on the nitrogen and oxygen atoms, respectively (Scheme 5.1-22), when treated with an alkyl halide and base (usually NaOH or KOH) in [BMIM][PF6] [51].
These reactions occur with similar rates to those carried out in dipolar aprotic solvents such as DMF or DMSO. An advantage of using the room-temperature ionic liquid for this reaction is that the lower reaction temperatures result in higher selectivities for substitution on the oxygen or nitrogen atoms. The by-product (sodium or potassium halide) of the reaction can be extracted with water and the ionic liquid recycled.
A quantitative study of the nucleophilic displacement reaction of benzoyl chloride with cyanide ion in [BMIM][PF6] was investigated by Eckert and co-workers [52]. The separation of the product, 1-phenylacetonitrile, from the ionic liquid was achieved by distillation or by extraction with supercritical CO2. The 1-phenylace- tonitrile was then treated with KOH in [BMIM][PF6] to generate an anion, which reacted with 1,4-dibromobutane to give 1-cyano-1-phenylcyclopentane (Scheme 5.1- 23). This was in turn extracted from the ionic liquid with supercritical CO2. These
|
|
|
Ph |
|
ONa |
[Bu4N]X |
O |
Ph |
|
or [Bu4P]X |
||||
|
|
OH |
||
+ Ph |
Br |
|
+ |
Scheme 5.1-21: The benzylation of sodium 2-naphthoxide with benzyl bromide in ammonium or phosphonium halide salts (X = Cl, Br).
OH |
|
[BMIM][PF6] |
O |
+ |
|
||
Br |
KOH |
|
|
|
|
|
+ KBr |
|
|
|
extract with water |
+ |
Br |
[BMIM][PF6] |
+ KBr |
|
KOH |
||
N |
|
|
N |
H
Scheme 5.1-22: Alkylation reactions in [BMIM][PF6].
