
Wasserscheid P., Welton T. - Ionic Liquids in Synthesis (2002)(en)
.pdf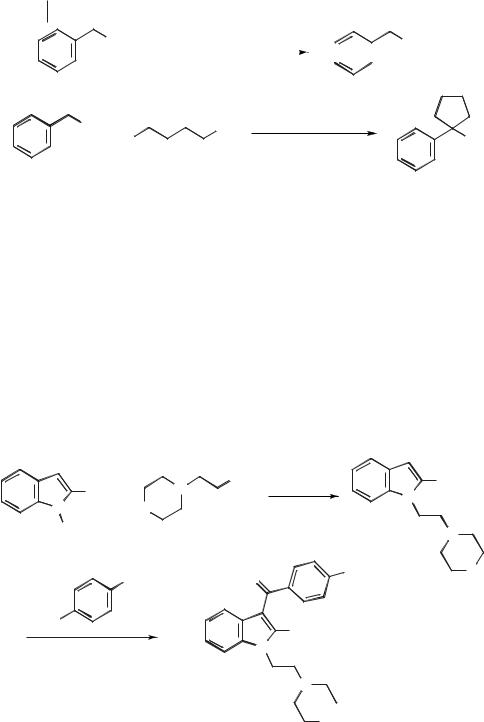
186 Martyn Earle
Cl |
+ KCN |
[BMIM][PF6] |
|
|
|
CN |
|
|
|
|
|
+ KCl |
|
|
|
|||||
|
|
|
|
|
|
|
CN |
Br [BMIM][PF6] / KOH |
+ Br |
CN |
Scheme 5.1-23. The reaction of cyanide with benzyl chloride to produce 1-phenylacetonitrile, and subsequent treatment with 1,4-dibromobutane.
reactions resulted in a build-up of KCl or KBr in the ionic liquid, which was removed by washing the ionic liquid with water.
As a demonstration of the complete synthesis of a pharmaceutical in an ionic liquid, Pravadoline was selected, as the synthesis combines a Friedel–Crafts reaction and a nucleophilic displacement reaction (Scheme 5.1-24) [53]. The alkylation of 2- methylindole with 1-(N-morpholino)-2-chloroethane occurs readily in [BMIM][PF6] and [BMMIM][PF6] (BMMIM = 1-butyl-2,3-dimethylimidazolium), in 95–99 % yields, with potassium hydroxide as the base. The Friedel–Crafts acylation step in [BMIM][PF6] at 150 °C occurs in 95 % yield and requires no catalyst.
Reactions involving organometallic reagents in neutral ionic liquid |
The addition of |
||||
organometallic reagents to carbonyl compounds is an important reaction in organ- |
|||||
ic chemistry, the Grignard reaction being one example of this. Procedures that |
|||||
|
|
Cl |
|
Base |
CH3 |
CH3 |
+ |
N |
|
N |
|
|
|
Ionic |
|||
N |
O |
. HCl |
|
|
|
H |
|
|
|
Liquid |
N |
|
|
|
|
||
|
|
|
|
|
|
|
OR |
|
O |
OR |
O |
|
|
|
|
||
ClOC |
|
|
|
CH3 |
|
|
|
|
|
|
|
Ionic Liquid |
|
|
|
N |
|
N
Pravadoline (R = CH3)
R = H, CH3  O
O
Scheme 5.1-24: The complete synthesis of Pravadoline in [BMIM][PF6].
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 187
O |
[BMIM][PF6] or |
OH |
|
||
|
|
|
H |
[BMIM][BF4] |
|
+ Sn |
4 16 h, 15 °C |
|
Scheme 5.1-25: Allylation of aldehydes in [BMIM][PF6] or [BMIM][BF4].
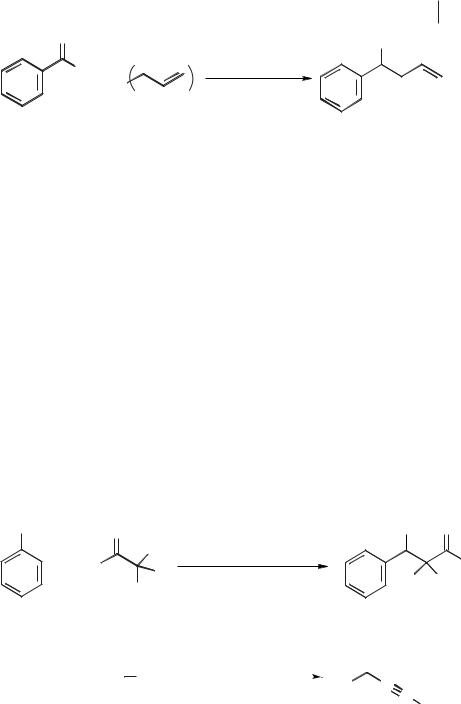
achieve similar results in ionic liquids are hence desirable. Gordon and McClusky [54] have reported the formation of homoallylic alcohols though the addition of allyl stannanes to aldehydes in the ionic liquids [BMIM][BF4] and [BMIM][PF6] (Scheme 5.1-25). It was found that the ionic liquid could be recycled and reused over several reaction cycles.
Kitazume and Kasai [55] have investigated the Reformatsky reaction in three ionic liquids. This reaction involves treatment of an α-bromo ester with zinc to give an α- zinc bromide ester, which in turn reacts with an aldehyde to give an addition product. An example is given in Scheme 5.1-26. Moderate to good yields (45–95 %) were obtained in ionic liquids such as [EDBU][OTf] for the reactions between ethyl bromoacetate or ethyl bromodifluoroacetate and benzaldehyde [55].
Reactions between aldehydes and alkynes to give propargyl alcohols are also described in Kitazume and Kasai’s paper [55]. Here, various aldehydes such as benzaldehyde or 4-fluorobenzaldehyde were treated with alkynes such as phenylethyne or pent-1-yne in three ionic liquids: [EDBU][OTf], [BMIM][PF6], and [BMIM][BF4] (Scheme 5.1-27). A base (DBU) and Zn(OTf)2 were required for the reaction to be effective; the yields were in the 50–70 % range. The best ionic liquid for this reaction depended on the individual reaction.
McCluskey et al. have also used [BMIM][BF4] as a solvent for the allylation of aldehydes and Weinreb amides [56]. Similar diastereoselectivities and similar or slightly lower yields were obtained in this ionic liquid, compared with reactions carried
CHO |
|
O |
|
OH |
O |
+ |
|
F |
Zn / [EDBU][OTf] |
|
OEt |
EtO |
|
|
|||
F |
50-60 °C |
|
|||
|
|
F |
F |
||
|
|
Br |
|
|
|
Scheme 5.1-26: The Reformatsky reaction in ionic liquids.
|
|
|
|
|
|
|
|
Zn(OTf)2 / ionic liquid |
|
OH |
|
R |
|
CHO + |
R’ |
|
C |
|
CH |
R |
|
C |
|
|
|
|
|
|
|||||||
|
|
DBU / 48 h, |
|
||||||||
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
room temperature |
|
|
C |
|
|
|
|
|
|
|
|
|
|
R ’ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5.1-27: The zinc triflate-catalyzed coupling of alkynes with aldehydes to give propargyl alcohols in an ionic liquid.
188 |
Martyn Earle |
|
|
|
|
|
|
|
|
|
Ph |
R |
|
|
|
|
|
|
N |
|
Ph |
R |
|
methanol or |
H |
|
|
|
syn |
|
||||
|
|
|
HO |
|||
N |
+ |
Sn |
[BMIM][BF4] |
|||
|
|
|||||
H |
H |
4 |
30 °C, 24 h |
|
+ |
|
|
Ph |
R |
||||
|
O |
|
|
|||
|
|
|
|
|
||
|
|
|
|
|
N |
|
|
|
|
|
H |
|
|
|
|
|
|
anti |
HO |
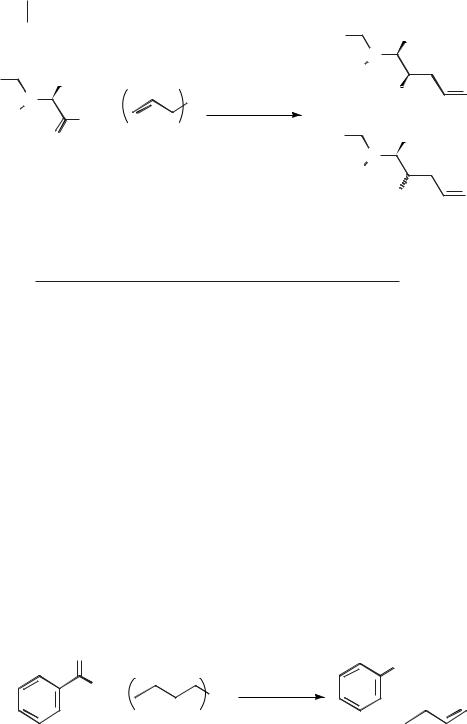
Scheme 5.1-28: The reaction between tetraallylstannane and an aldehyde in methanol or [BMIM][BF4].
Table 5.1-2: The yields and selectivities for the reaction shown in Scheme 5.1-28.
R |
syn- : anti- |
% Yield |
% Yield |
d.e. (%) |
|
|
[BMIM][BF4] |
methanol |
|
CH3 |
82:18 |
72 |
87 |
64 |
CH(CH3)2 |
93:7 |
70 |
74 |
86 |
PhCH2 |
93:7 |
73 |
82 |
86 |
out in methanol (Scheme 5.1-28). The lower yield assigned to the reaction in the ionic liquid (see Table 5.1-2) is thought to be due to difficulty in extracting the product from the ionic liquid.
Ionic liquids such as [BMIM][BF4] and [EMIM][PF6] have been used in the trialkylborane reduction of aldehydes to alcohols (Scheme 5.1-29) [57]. In the reduction of benzaldehyde with tributylborane, similar yields (90–96 %) were obtained for the ionic liquids [EMIM][BF4], [EMIM][PF6], [BMIM][BF4], and [BMIM][PF6]. The effect of electron-releasing and electron-withdrawing groups on the aromatic aldehyde were investigated. In general, electron-withdrawing groups such as halogen give near quantitative yields, but electron-releasing groups such as methoxy reduced the reaction rate and yield.
Miscellaneous reactions in neutral ionic liquids Kitazume et al. have also investigated the use of [EDBU][OTf] as a medium in the formation of heterocyclic compounds [58]. Compounds such as 2-hydroxymethylaniline readily condense with
O
|
|
|
CH2OH |
H + |
[EMIM][PF6] |
|
|
B |
°C |
|
|
3 |
100 |
+ |
|
Scheme 5.1-29: The reduction of benzaldehyde in [EMIM][PF6].
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 189
OH |
|
|
O |
O |
|
|
|
||
|
|
|
[EDBU][OTf] |
|
|
+ |
H |
|
|
|
|
N |
||
NH2 |
|
|
||
|
|
|
||
|
|
|
|
H
Scheme 5.1-30: The formation of 2-phenylbenzoxazine in [EDBU][OTf].
benzaldehyde to give the corresponding benzoxazine (Scheme 5.1-30). The product of the reaction is readily extracted with solvents such as diethyl ether, and the ionic liquid can be recycled and reused.
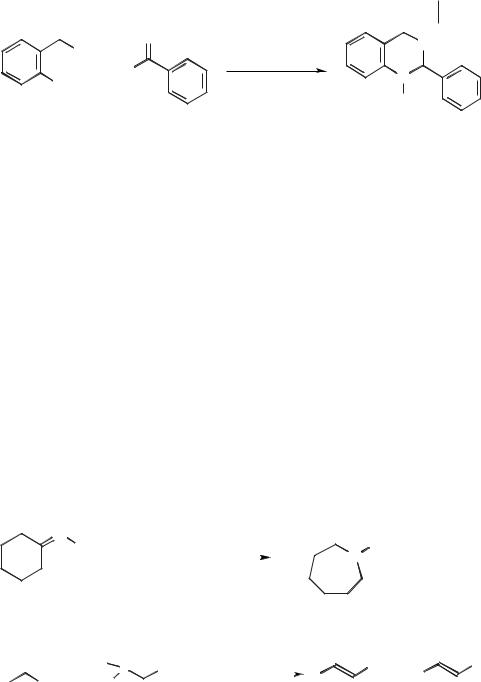
Beckmann rearrangements of several ketoximes were performed in room-tem- perature ionic liquids based on 1,3-dialkylimidazolium or alkylpyridinium salts containing phosphorus compounds (such as PCl5) by Deng and Peng [59] (Scheme 5.1-31, BP = 1-butylpyridinium). Turnover numbers of up to 6.6 were observed, but the authors did not mention whether the ionic liquid could be reused.
The first examples of Horner–Wadsworth–Emmons reactions have been reported by Kitazume and Tanaka [60]. Here the ionic liquid [EDBU][OTf] was used in the synthesis of α-fluoro-α,β-unsaturated esters (Scheme 5.1-32). It was found that when K2CO3 was used as a base, the E isomer was the major product and that when DBU was used as a base, the Z isomer was the major product. The reaction was also performed in [EMIM][BF4] and [EMIM][PF6], but gave lower yields than with [EDBU][OTf] [60].
Davis and co-workers have carried out the first examples of the Knoevenagel condensation and Robinson annulation reactions [61] in the ionic liquid [HMIM][PF6] (HMIM = 1-hexyl-3-methylimidazolium) (Scheme 5.1-33). The Knoevenagel condensation involved the treatment of propane-1,3-dinitrile with a base (glycine) to generate an anion. This anion added to benzaldehyde and, after loss of a water molecule, gave 1,1-dicyano-2-phenylethene. The product was separated from the ionic liquid by extraction with toluene.
N |
|
O |
||
PCl5, 2 h, 80 °C |
|
|
H |
|
OH |
|
|
||
|
|
|||
|
|
|
|
N |
|
[BP][BF4] |
|
|
|
|
|
|
|
|
Scheme 5.1-31: The Beckmann rearrangement in ionic liquids.
|
O |
O |
|
|
R |
|
H |
||||||||||
|
CO2Et DBU or K2CO3 |
|
|
|
|
|
|
||||||||||
|
EtO |
|
|
|
|
|
|
F |
|
|
|
F |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
+ |
P |
+ |
||||||||||||
|
|
|
H |
|
R |
||||||||||||
R |
|
|
H |
EtO |
|
|
[EDBU][OTf] |
|
|
|
|
||||||
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
F |
|
|
|
CO2Et |
|
|
CO2Et |
||||
|
|
|
|
|
|
|
|
|
|
E Isomer |
|
Z isomer |
|||||
Scheme 5.1-32: The Horner-Wadsworth-Emmons reaction in an ionic liquid.

190 |
Martyn Earle |
|
|
|
|
||
|
|
|
|
|
|
O |
|
|
|
|
|
O |
|
NH2 |
CN |
|
|
|
|
|
|
||
NC |
CN |
+ |
|
|
|
HO |
|
H |
|
|
|
|
|||
|
|
|
|
|
[HMIM][PF6] |
CN |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
room temperature |
|
O |
O |
|
|
|
O |
O |
O |
|
|
|
|
||||
|
|
|
|
NaOH |
|
||
|
|
+ |
|
|
|
|
|
|
|
|
|
|
EtO |
|
|
EtO |
|
|
|
|
|
[HMIM][PF6] |
|
|
|
|
|
|
|
heat |
|
Scheme 5.1-33: The Knoevenagel condensation and the Robinson annulation in [HMIM][PF6].
The Robinson annulation of ethyl acetoacetate and trans-chalcone proceeded smoothly to give 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone in 48 % yield. The product was separated from the ionic liquid by solvent extraction with toluene. In both these reactions, the ionic liquid [HMIM][PF6] was recycled and reused with no reduction in the product yield.
Deng and Peng have found that certain ionic liquids catalyze the Biginelli reaction [62]. Usually, this reaction is catalyzed by Lewis acids such as InCl3, [Fe(H2O)6]Cl3, or BF3.O(C2H5)2, or by acid catalysts such as Nafion-H. The reaction was found to give yields in the 77–99 % range in the ionic liquids [BMIM][PF6] or [BMIM][BF4] for the examples in Scheme 5.1-34. The reaction fails if there is no ionic liquid present or in the presence of tetrabutylammonium chloride.
Singer and Scammells have investigated the γ-MnO2 oxidation of codeine methyl ether (CME) to thebaine in the ionic liquid [BMIM][BF4] [63]. The ionic liquid was used in different ways and with mixed results (Scheme 5.1-35). For example, the oxidation of CME in the ionic liquid gave 38 % yield after 120 hours. A similar reaction under biphasic conditions (with diethyl ether) gave a 36 % yield of thebaine. This reaction gave a 25 % yield of thebaine when carried out in tetrahydrofuran
|
|
|
|
|
|
|
|
|
|
|
ionic |
O |
|
R |
|
||||
|
O |
O |
O |
|
|
|
|
|
|
|
H |
||||||||
|
liquid |
|
|
|
|
|
|
|
|||||||||||
|
CHO |
|
|
|
|
|
|
|
|
|
R1 |
|
|
N |
|
||||
R |
+ |
|
|
+ |
|
|
|
|
|
R1 |
100 °C, 1 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
H2N |
NH2 |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
H3C |
|
N |
O |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
||
|
Scheme 5.1-34: |
The Biginelli reaction in an ionic liquid. R = C6H5, 4-(H3CO)-C6H4, 4-Cl-C6H4, |
|
||||||||||||||||
|
4-(O2N)-C6H4, C5H11. R1 = OC2H5, CH3. |
|
|
|
|
|
|
|
|
|
|||||||||
|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 191 |
||
H3CO |
|
|
H3CO |
O |
|
MnO2 |
O |
|
|
||
|
NCH3 |
[BMIM][BF4] |
NCH3 |
H3CO |
|
|
H3CO |
Scheme 5.1-35: |
The oxidation of CME to thebaine in [BMIM][BF4]. |
|
|
(THF). The authors found that the yield could be increased to 95 % by sonication of the reaction vessel, for the reaction in THF. The ionic liquid was then used to extract the manganese by-products and impurities from an ethyl acetate solution of the product [63].
A novel use of the salt [BMIM][PF6] is to enhance microwave absorption and hence accelerate the rate of a reaction. Ley found that [BMIM][PF6] enhanced the rate of the microwave-promoted thionation of amides by a polymer-supported thionating agent [64].
Hardacre et al. have developed a procedure for the synthesis of deuterated imidazoles and imidazolium salts [65]. The procedure involves the platinumor palla- dium-catalyzed deuterium exchange of 1-methyl-d3-imidazole with D2O to give 1- methylimidazole-d6, followed by treatment with a deuterated alkyl halide.
5.1.2
Acid-Catalyzed Reactions
5.1.2.1Electrophilic substitutions and additions
This section deals with Brønsted acid and Lewis acid catalyzed reactions, excluding Friedel–Crafts reactions, but including reactions such as nitrations, halogenations, and Claisen rearrangements. Friedel–Crafts reactions are discussed in the subsequent Sections 5.1.2.2 and 5.1.2.3.
The first example of an electrophilic nitration in an ionic liquid was performed by Wilkes and co-workers [66]. A number of aromatic compounds were nitrated with KNO3 dissolved in chloroaluminate(III) ionic liquids. A number of nitration reactions have also been carried out by Laali et al. [67]. The reactions of nitrates, preformed nitronium salts, and alkyl nitrates with aromatic compounds have been performed in a wide range of ionic liquids. Reactions between toluene and [NO2][BF4], for example, have been performed with varying degrees of success in [EMIM]Cl, [EMIM][AlCl4], [EMIM][Al2Cl7], [EMIM][BF4], [EMIM][PF6], and [EMIM][OTf]. Of these, the reaction in [EMIM][BF4] (Scheme 5.1-36) gave the best yield (71 %, o:p ratio = 1.17:1), but only after the imidazolium ring had undergone nitration (Figure 5.1-3).
192 Martyn Earle
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
CH3 |
||||||
|
|
|
|
|
CH3 excess [NO |
|
|
|
|
|
|
||||||||||
|
|
|
|
|
][BF ] |
|
|
|
|
|
|
|
NO2 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
2 |
4 |
|
|
|
|
+ |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
[EMIM][BF4] |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NO2 |
|
|
|
|
|||
Scheme 5.1-36: |
The nitration of toluene with [NO2][BF4] in [EMIM][BF4]. |
|
|
|
|
||||||||||||||||
|
|
|
|
|
NO2 |
|
|
|
Figure 5.1-3: |
The nitroimidazolium ionic liquid. |
|||||||||||
|
|
|
|
|
|
|
[BF4]- |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
H3C |
N |
+ |
|
|
N |
C2H5 |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other methods of nitration that Laali investigated were with isoamyl nitrate in combination with a Brønsted or Lewis acid in several ionic liquids, with [EMIM][OTf] giving the best yields (69 %, 1.0:1.0 o:p ratio). In the ionic liquid [HNEt(iPr)2] [CF3CO2] (m.p. = 92–93 °C), toluene was nitrated with a mixture of [NH4][NO3] and trifluoroacetic acid (TFAH) (Scheme 5.1-37). This gave ammonium trifluoroacetate [NH4][TFA] as a by-product, which could be removed from the reaction vessel by distillation (sublimation).
Wilkes and co-workers have investigated the chlorination of benzene in both acidic and basic chloroaluminate(III) ionic liquids [66]. In the acidic ionic liquid [EMIM]Cl/AlCl3 (X(AlCl3) > 0.5), the chlorination reaction initially gave chlorobenzene, which in turn reacted with a second molecule of chlorine to give dichlorobenzenes. In the basic ionic liquid, the reaction was more complex. In addition to the
CH3
CH3 |
ONO2 |
BF3OEt2 or TfOH |
+ |
||
|
|
[EMIM][OTf] |
|
|
|
NO2
OCH3
OCH3
[NH4][NO3] / TFAH
|
|
|
|
99 % NMR yield, |
|
[HNEt(Pri)2] [CF3CO2] |
|||||
|
|
|
p- : o- ratio = |
||
|
|
|
74 : 26 |
||
|
|
|
NO2 |
||
Scheme 5.1-37: Aromatic nitration reactions in ionic liquids.
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids |
|
193 |
|||||
|
|
|
|
Cl |
|
|
|
|
Cl |
|
Cl |
||||
|
|
|
|
|
|
|
Cl |
Cl2 |
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
||
[EMIM]Cl-AlCl3 (X > 0.5)
|
|
|
Cl |
|
|
|
Cl |
Cl |
|
Cl |
|
|
|
|
|
||
Cl2 |
|
Cl |
Cl |
Cl |
|
|
+ |
+ |
|
||
[EMIM]Cl-AlCl3 |
(X < 0.5) |
|
|||
Cl |
Cl |
Cl |
|||
|
|
||||
|
|
Cl |
|
Cl |
Scheme 5.1-38: The chlorination of benzene in acidic and basic chloroaluminate ionic liquids.
formation of chlorobenzene, addition products of chlorine and benzene were observed. These addition products included various isomers of tetrachlorocyclohexene and hexachlorocyclohexane (Scheme 5.1-38).
Another common reaction is the chlorination of alkenes to give 1,2-dihaloalka- nes. Patell et al. reported that the addition of chlorine to ethene in acidic chloroaluminate(III) ionic liquids gave 1,2-dichloroethane [68]. Under these conditions, the imidazole ring of imidazolium ionic liquid is chlorinated. Initially, the chlorination occurs at the 4- and 5-positions of the imidazole ring, and is followed by much slower chlorination at the 2-position. This does not affect the outcome of the alkene chlorination reaction and it was found that the chlorinated imidazolium ionic liquids are excellent catalysts for the reaction (Scheme 5.1-39).
In an attempt to study the behavior and chemistry of coal in ionic liquids, 1,2- diphenylethane was chosen as a model compound and its reaction in acidic pyridinium chloroaluminate(III) melts ([PyH]Cl/AlCl3 was investigated [69]. At 40 °C, 1,2-diphenylethane undergoes a series of alkylation and dealkylation reactions to give a mixture of products. Some of the products are shown in Scheme 5.1-40. Newman also investigated the reactions of 1,2-diphenylethane with acylating agents such as acetyl chloride or acetic anhydride in the pyridinium ionic liquid [70] and with alcohols such as isopropanol [71].
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl |
|
|
|
|
Cl |
|
[Al2Cl7]- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H5C2 N + N CH3 |
|
|
|
|
H5C2 N |
|
+ N CH3 |
||||||||||||
|
|
[Al2Cl7]- |
|
|
Cl |
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
Cl |
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Cl2 |
|
|
C l |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Scheme 5.1-39: The chlorination of ethene to give 1,2-dichloroethane.
194 Martyn Earle
PyHC l-AlCl3 |
|
|
|
|
|
|
|
|
(X = 0.67) |
|
|
|
+ |
|
|
+ |
|
|
|
|
|
|
|
|||
40 °C |
||||||||
|
|
|
|
|
|
|
+
+
Scheme 5.1-40: The reaction of 1,2-diphenylethane with PyHCl/AlCl3 (X(AlCl3) = 0.67).
Kitazume and Zulfiqar have investigated the Claisen rearrangement of several aromatic allyl ethers in ionic liquids, catalyzed by scandium(III) trifluoromethanesulfonate [72]. The reaction initially gave the 2-allylphenol but this reacted further to give 2-methyl-2,3-dihydrobenzo[b]furan (Scheme 5.1-41). The yields in this reaction were highly dependant on the ionic liquid chosen, with [EDBU][OTf] giving the best yields (e.g., 91 % for R = 6-CH3). Reactions in [BMIM][BF4] and [BMIM][PF6] gave low yields (9–12 %).
In order to confirm that 2-allylphenol was an intermediate in the reaction, the authors subjected 2-allylphenol to the same reaction conditions and found that it rearranged to give 2-methyl-2,3-dihydrobenzo[b]furan. On treatment of 2-methyl-2- propenyl phenyl ether (Scheme 5.41) under similar conditions, 2,3-diisopropylben- zo[b]furan was isolated in 15 % yield. A mechanistic scheme is given by the authors (Scheme 5.1-42). It involves the Claisen rearrangement of 2-methyl-2-propenyl phenyl ether to 2-(2-methyl-2-propenyl)-phenol, followed by a transalkylation of a 2-methylpropenyl group to the phenyl OH group. This undergoes further rearrangements and cyclization to give the 2,3-diisopropylbenzo[b]furan [72].
Lee et al. have investigated the Lewis acid-catalyzed three-component synthesis of α-amino phosphonates [73]. This was carried out in the ionic liquids [BMIM][PF6],
O |
5 mol % Sc(OTf)3 |
OH |
O |
|
|||
|
200 °C / 10 hours |
|
|
|
[EDBU][OTf] |
|
|
|
R |
R |
R |
|
|
Scheme 5.1-41: Claisen rearrangements of several phenyl allyl ethers (R = H, 4-CH3, 6-CH3).

|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids |
195 |
||
|
|
|
|
|
|
|
|||
O |
OH |
|
|
|
|
||||
O
OH |
OH |
H+
OH |
+ |
O |
O |
|
|
Scheme 5.1-42: Proposed mechanism for the formation of 2,3-diisopropylbenzo[b]furan.
[BMIM][OTf], [BMIM][BF4], and [BMIM][SbF6], and the results were compared with a similar reaction carried out in dichloromethane (Scheme 5.1-43).
Lee found that the reaction gave good yields (70–99 %) in the ionic liquids [BMIM][PF6], [BMIM][OTf], and [BMIM][SbF6] with Lewis acids such as Yb(OTf)3, Sc(OTf)3, Dy(OTf)3, Sm(OTf)3, and InCl3. The reaction was also performed in [BMIM][PF6] or dichloromethane with Sm(OTf)3 as the catalyst. The ionic liquid reaction gave a yield of 99 %, compared with 70 % for the reaction in dichloromethane [73].
CHO |
NH2 |
|
|
O |
10 mol. % |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
HN |
||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
H |
|
|
|
|
OC2H5 |
catalyst |
|
|
|
|
|
|
|
+ |
|
|
+ |
|
P |
|
|
|
|
|
|
OC2H5 |
|||||
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
ionic liquid |
|
|
|
|
P |
||
|
|
|
|
|
|
OC2H5 |
|||||||||||
|
|
|
|
|
|
20 °C |
|
|
|
|
|
OC2H5 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ H2O |
|||
Scheme 5.1-43: Three-component reaction of benzaldehyde, aniline, and diethyl phosphonate in ionic liquids, catalyzed by lanthanide triflates and indium(III) chloride.
