
Wasserscheid P., Welton T. - Ionic Liquids in Synthesis (2002)(en)
.pdf
196 Martyn Earle
5.1.2.2Friedel–Crafts alkylation reactions
Friedel–Crafts reactions have been studied in detail by Olah [74, 75]. These reactions result in the formation of carbon–carbon bonds and are catalyzed by strong Brønsted or Lewis acids.
The Friedel–Crafts alkylation reaction usually involves the interaction of an alkylation agent such as an alkyl halide, alcohol, or alkene with an aromatic compound, to form an alkylated aromatic compound (Scheme 5.1-44).
It should be noted that Scheme 5.1-44 shows idealized Friedel–Crafts alkylation reactions. In practice, there are a number of problems associated with the reaction. These include polyalkylation reactions, since the products of a Friedel–Crafts alkylation reaction are often more reactive than the starting material. Also, isomerization and rearrangement reactions can occur, and can result in a large number of products [74, 75]. The mechanism of Friedel–Crafts reactions is not straightforward, and it is possible to propose two or more different mechanisms for a given reaction. Examples of the typical processes occurring in a Friedel–Crafts alkylation reaction are given in Scheme 5.1-45 for the reaction between 1-chloropropane and benzene.
The chemical behavior of Franklin acidic chloroaluminate(III) ionic liquids (where X(AlCl3) > 0.50) [6] is that of a powerful Lewis acid. As might be expected, it catalyzes reactions that are conventionally catalyzed by aluminium(III) chloride, without suffering the disadvantage of the low solubility of aluminium(III) chloride in many solvents.
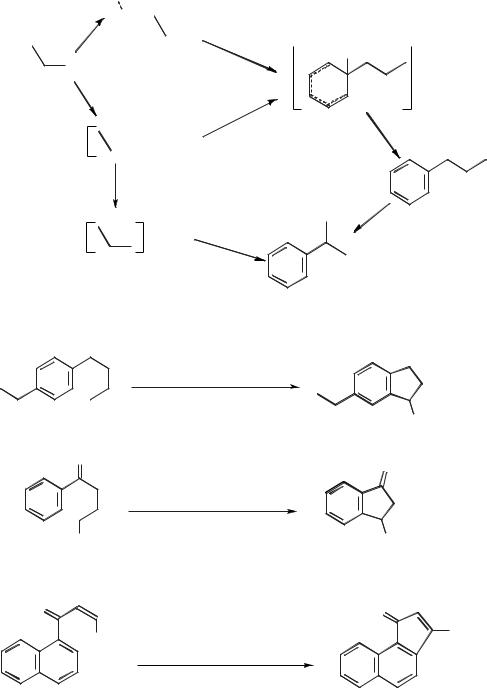
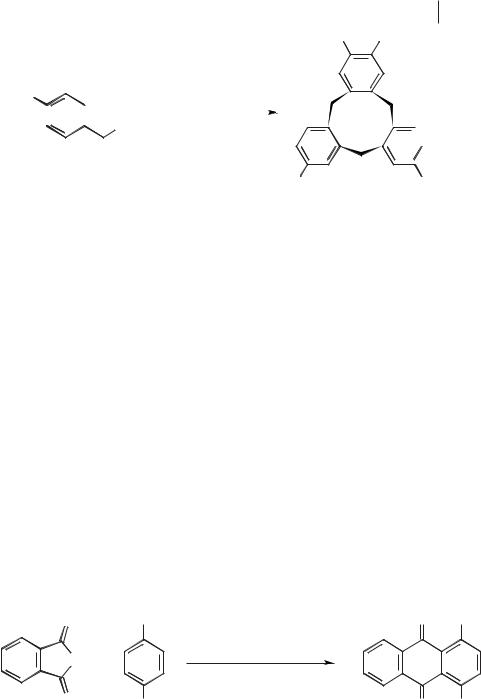
The first examples of alkylation reactions in molten salts were reported in the 1950’s. Baddeley and Williamson performed a number of intramolecular cyclization reactions [76] (Scheme 5.1-46), carried out in mixtures of sodium chloride and aluminium chloride. The reactions were run at below the melting point of the pure salt, and it is presumed that the mixture of reagents acts to lower the melting point.
Baddeley also investigated the cyclization of alkenes in the NaCl/AlCl3 molten salt. An example is given in Scheme 5.1-47 [77].
Mendelson et al. [78] also investigated a number of cyclization reactions. One of these involved the cyclodehydration of N-benzylethanolamine chloride in a molten salt derived from AlCl3 and NH4Cl (X(AlCl3) = 0.73). This gave rise to the corresponding tetrahydroisoquinoline in 41–80 % yield, as shown in Scheme 5.1-48.
H |
|
|
|
|
R |
||||
+ X |
|
R |
|
|
|
|
+ H |
|
X |
|
|
|
|
|
|
||||
|
|||||||||
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
+ |
R |
R |
+ H X |
|
|||
|
|
|
Scheme 5.1-44: The Friedel-Crafts alkylation reaction (R = alkyl, X = leaving group).
|
|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids |
197 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl3Al |
|||||||||
|
|
Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
AlCl3 |
|||||||||
Cl |
|
|
|
|
|
Ph-H |
|
|
|
|
|
|
|
|
|
|
|
H |
|||||
|
|
|
|
|
|
|
|
||||
[AlCl4]-
+
AlCl3
+ |
|
|
|
[AlCl4]- |
Ph-H |
- HCl |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[AlCl4]- |
AlCl3 / H+ |
Ph-H |
|
+ |
-HCl |
|
Scheme 5.1-45: The reaction between 1-chloropropane and benzene under Friedel-Crafts conditions.
NaCl-AlCl3 (X = 0.82) /
100 °C, 1 hour
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
Cl |
|
|
|
CH3 |
|||
O |
||||||||
O
O
NaCl-AlCl3 (X = 0.77) /
100 °C, 1 hour
Br |
CH3 |
Scheme 5.1-46: The intramolecular cyclization of alkyl chlorides and bromides.
O |
|
O |
CO |
H |
CO2H |
NaCl-AlCl3 (X = 0.75) / |
||
2 |
|
115 °C, 1 hour |
|
|
Scheme 5.1-47: The intramolecular cyclization of an alkene.
198 |
|
Martyn Earle |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
R1 |
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
R2 |
|
|
|
[NH4]Cl-AlCl3 |
|
|
|
H |
|
||||||||||||
|
+ |
OH |
|
|
|
R2 |
|
|
|
|
|||||||||||
|
|
|
|
|
|
N+ |
|
|
|
||||||||||||
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
N |
|
|
Cl- |
(X = 0.73) |
|
|
|
|
|
|
|
H |
Cl- |
|||
|
|
|
|
|
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
R3 |
R4 |
|
|
|
|
|
|
R3 |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
R4 |
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
Scheme 5.1-48: |
The cyclodehydration of N-benzylethanolamine chloride. |
|
|
|
|
|
|||||||||||||
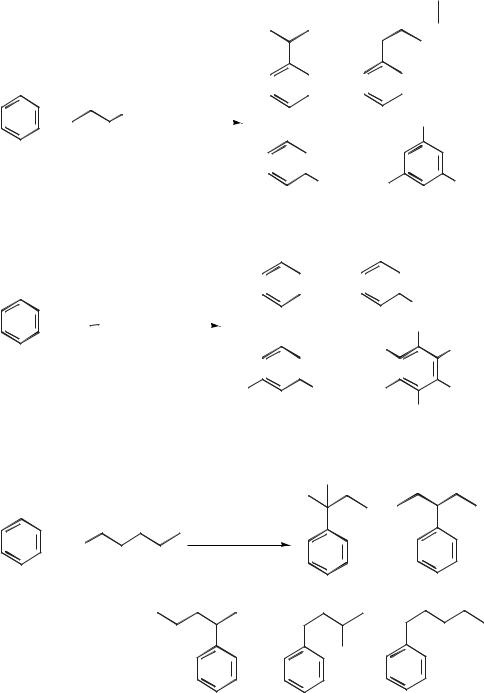
Boon et al. investigated the reactions of benzene and toluene in room-tempera- ture ionic liquids based on [EMIM]Cl/AlCl3 mixtures [79]. The reactions of various alkyl chlorides with benzene in the ionic liquid [EMIM]Cl/AlCl3 (X(AlCl3) = 0.60 or 0.67) were carried out, and the product distributions are given in Table 5.1-3 and Scheme 5.1-49. The methylation of benzene with methyl chloride proceeds to give predominantly dimethylbenzene (xylenes) and tetramethylbenzene, with about 10 % hexamethylbenzene. In the propylation of benzene with 1-chloropropane, not only does polyalkylation occur, but there is a considerable degree of isomerization of the n-propyl group to the isopropyl isomer (Scheme 5.1-49). In the butylation, complete isomerization of the butyl side chain occurs, to give only sec-butyl benzenes.
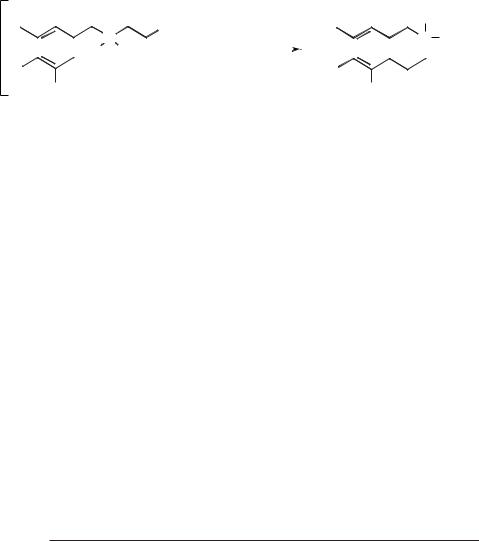
Piersma and Merchant have studied the alkylation of benzene with various chloropentanes in [EMIM]Cl/AlCl3 (X(AlCl3) = 0.55) [80]. Treatment of 1-chloropen- tane with benzene gave a mixture of products, with only a 1 % yield of the unisomerized n-pentylbenzene. The major products of the reaction had all undergone isomerization (Scheme 5.1-50).
Details of two related patents for the alkylation of aromatic compounds with chloroaluminate(III) ionic or chlorogallate(III) ionic liquid catalysts have become available. The first, by Seddon and co-workers [81], describes the reaction between ethene and benzene to give ethylbenzene (Scheme 5.1-51). This is carried out in an
Table 5.1-3: The products from the reactions between alkyl chlorides and benzene in [EMIM]Cl/AlCl3 (X(AlCl3) = 0.60 or 0.67).
R -Cl |
X |
R-Cl : C6H6 |
: |
Mono- |
Di- |
Tri- |
Tetra- |
Penta- |
Hexa- |
|
|
|
|
IL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Methyla |
0.67 |
xs : 1 : 1 |
|
1.5 |
58.5 |
1.5 |
26.8 |
1.4 |
10.2 |
|
Ethyla |
0.67 |
xs : 1 : 1 |
|
11.5 |
10.8 |
33.4 |
24.4 |
|
1.5 |
|
n-Propylb |
0.60 |
1.25 |
: 1.25 : |
1 |
24.8 |
19.9 |
55.3 |
|
|
|
n-Butylb,c |
0.60 |
1.33 |
: 1.33 : |
1 |
25.0 |
26.3 |
48.7 |
|
|
|
Cyclohexyl |
0.60 |
10 |
: 10 : 1 |
|
35.0 |
30.0 |
34.4 |
|
|
|
Benzyld |
0.60 |
0.78 |
: 1.17 : |
1 |
50.0 |
34.5 |
15.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a At reflux temperature of alkyl halide. b Room temperature in dry-box. c Only sec-butyl products formed. d Tar formed, only a small amount of alkylated product isolated.
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 199
|
|
|
[EMIM]Cl-AlCl3 |
|
|
|
|
|
+ |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
+ |
|
Cl |
(X = 0.67) |
|
C3H7 |
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
25 °C |
+ |
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C3H7 |
H7C3 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
C H3 |
|
C H3 |
|
|
||||||
|
[EMIM]Cl-AlCl3 |
|
|
|
|
|
|
+ |
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
+ H |
C Cl |
(X = 0.60) |
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
C H |
|
|
|
|
|
||||||||
3 |
-12 |
°C |
3 |
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
H3C |
|
|
||||||||||
|
|
|
|
+ |
|
|
|
|
|
|
|
|
+ |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
H3C |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
H3C |
|
|
|
|
|
CH3 |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
C H3 |
|
|
|
|
|
|||||
Scheme 5.1-49: The alkylation of benzene with methyl chloride or n-propyl chloride in an ionic liquid.
|
[EMIM]Cl-AlCl3 |
+ Cl |
(X = 0.55) |
+ |
|
|
78.5 % |
+ |
+ |
+ |
C3H7
C3H7
CH3
C H3
CH3
CH3
C H3
13.5 %
4.5 % |
2.5 % |
1.0 % |
Scheme 5.1-50: The reaction between 1-chloropentane and benzene in [EMIM]Cl/AlCl3 (X(AlCl3) = 0.55).

200 Martyn Earle
N + N  Cl-/ MCl3
Cl-/ MCl3
+ H2C  CH2
CH2
M = Al, Ga
Scheme 5.1-51: The alkylation of aromatic compounds in chloroaluminate(III) or chlorogallate(III) ionic liquids.
acidic ionic liquid based on an imidazolium cation, and is claimed for ammonium, phosphonium, and pyridinium cations. The anion exemplified in the patent is a chloroaluminate(III) and a claim is made for chlorogallate(III) anions and various mixtures of anions.
The second patent, by Wasserscheid and co-workers [82], also describes the reaction of benzene with ethene in ionic liquids, but exemplifies a different ionic liquid suitable for this reaction (Scheme 5.1-52).
The production of linear alkyl benzenes (LABs) is carried out on a large scale for the production of surfactants. The reaction involves the reaction between benzene and a long-chain alkene such as dodec-1-ene and often gives a mixture of isomers. Greco et al. have used a chloroaluminate(III) ionic liquid as a catalyst in the preparation of LABs [83] (Scheme 5.1-53).
[Et3NH]Cl - MCl3
+ H2C  CH2
CH2
M = Al, Ga
Scheme 5.1-52: The reaction between benzene and ethene in a triethylammonium ionic liquid.
[(CH3)3NH]Cl-AlCl3 (X = 0.66) + 






46 %
19 %
other isomers = 22 %
12 %
Scheme 5.1-53: The reaction between dodec-1-ene and benzene with an ionic liquid as a catalyst.

5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 201
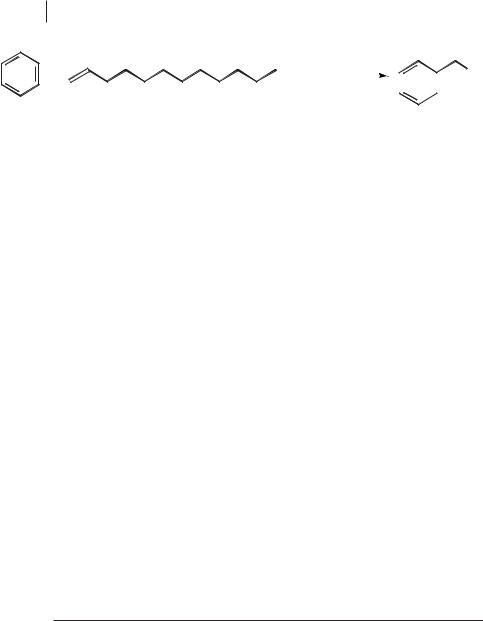
Keim and co-workers have carried out various alkylation reactions of aromatic compounds in ionic liquids substantially free of Lewis acidity [84]. An example is the reaction between benzene and decene in [BMIM][HSO4], which was used together with sulfuric acid as the catalyst (Scheme 5.1-54). These authors have also claimed that these acid-ionic liquids systems can be used for esterification reactions.
The methodology of a Lewis acid dissolved in an ionic liquid has been used for Friedel–Crafts alkylation reactions. Song [85] has reported that scandium(III) triflate in [BMIM][PF6] acts as an alkylation catalyst in the reaction between benzene and hex-1-ene (Scheme 5.1-55).
The ionic liquids that were found to give the expected hexylbenzenes were [BMIM][PF6], 1-pentyl-3-methylimidazolium hexafluorophosphate, [HMIM][PF6], [EMIM][SbF6], and [BMIM][SbF6]. The reaction did not succeed in the corresponding tetrafluoroborate or trifluoromethanesulfonate ionic liquids. For the successful reactions, conversions of 99 % of the hexene into products occurred, with 93–96 % of the products being the monoalkylated product (Scheme 5.1-55). The authors noted that the successful reactions all took place in the hydrophobic ionic liquids. It should be noted that the [PF6]– and [SbF6]– ions are less stable to hydrolysis reactions, resulting in the formation of HF, than the [BF4]– or [OTf]– ions, and so the possibility of these reactions being catalyzed by traces of HF cannot be excluded [86].
The alkylation of a number of aromatic compounds through the use of a chloroaluminate(III) ionic liquid on a solid support has been investigated by Hölderich and co-workers [87, 88]. Here the alkylation of aromatic compounds such as benzene, toluene, naphthalene, and phenol with dodecene was performed using the ionic liquid [BMIM]Cl/AlCl3 supported on silica, alumina, and zirconia. With benzene, monoalkylated dodecylbenzenes were obtained (Scheme 5.1-56).
N + N |
[HSO4]- |
+ |
(CH2)7CH3 |
H2SO4 |
|
|
+ isomers |
Scheme 5.1-54: The sulfuric acid-catalyzed alkylation of benzene in a hydrogensulfate ionic liquid.
Sc(OTf)3 (0.2 eq.) |
|
ionic liquid |
|
+ |
+ |
12 hours, 20 °C |
Scheme 5.1-55: The alkylation of benzene with hex-1-ene, catalyzed by scandium(III) triflate in ionic liquids.
202 Martyn Earle
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
+ |
|
|
|
|
|
|
[BMIM]Cl-AlCl3 |
|
|
|
|
R2 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
solid support |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 = CH , |
|
R2 |
= (CH ) CH |
3 |
2-phenyldodecane |
|
|
|
|
|
||
3 |
|
|
2 9 |
|
|
|
|
|
|
|
||
R1 = CH CH , |
R2 |
= (CH ) CH |
3-phenyldodecane |
|
|
|
|
|
||||
2 |
3 |
|
2 |
8 |
3 |
|
|
|
|
|
|
|
R1 = (CH2)2CH3, R2 = (CH2)7CH3 |
4-phenyldodecane |
|
|
|
|
|
||||||
R1 = (CH2)3CH3, R2 |
= (CH2)6CH3 |
5-phenyldodecane |
|
|
|
|
|
|||||
R1 = (CH2)4CH3, R2 |
= (CH2)5CH3 |
6-phenyldodecane |
|
|
|
|
|
|||||
Scheme 5.1-56: The alkylation of benzene with dodecene with an ionic liquid on a solid support.
The product distribution in the reaction of benzene with dodecene was determined for a number of catalysts (Table 5.1-4). As can be seen, the reaction with the zeolite H-Beta gave predominantly the 2-phenyldodecane, whereas the reaction in the pure ionic liquid gave a mixture of isomers, with selectivity similar to that of aluminium chloride. The two supported ionic liquid reactions (H-Beta / IL and T 350 / IL) again gave product distributions similar to aluminium(III) chloride (T350 is a silica support made by Degussa).
Raston has reported an acid-catalyzed Friedel–Crafts reaction [89] in which compounds such as 3,4-dimethoxyphenylmethanol were cyclized to cyclotriveratrylene (Scheme 5.1-57). The reactions were carried out in tributylhexylammonium bis(trifluoromethanesulfonyl)amide [NBu3(C6H13)][(CF3SO2)2N] with phosphoric or p- toluenesulfonic acid catalysts. The product was isolated by dissolving the ionic liquid/catalyst in methanol and filtering off the cyclotriveratrylene product as white crystals. Evaporation of the methanol allowed the ionic liquid and catalyst to be regenerated.
Table 5.1-4: The product distribution dependency on the catalyst used for the reaction of benzene with dodecene. IL = [BMIM]Cl/AlCl3 (X(AlCl3) = 0.6), Temperature = 80 °C, with 6 mol% catalyst and benzene to dodecene ratio = 10:1.
Catalyst |
2-Phenyl |
3-Phenyl |
4-Phenyl |
5-Phenyl |
6-Phenyl |
|
dodecane |
dodecane |
dodecane |
dodecane |
dodecane |
|
|
|
|
|
|
AlCl3 |
46.4 |
19.4 |
12.7 |
12.1 |
9.5 |
IL (X(AlCl3) = 0.6) |
36.7 |
19.0 |
15.0 |
15.5 |
13.8 |
T 350 / IL |
42.9 |
22.8 |
13.0 |
11.8 |
9.4 |
H-Beta |
75.7 |
19.0 |
3.8 |
1.1 |
0.4 |
H-Beta / IL |
43.9 |
21.2 |
12.4 |
12.0 |
10.5 |
|
|
|
|
|
|
5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 203
H3CO
|
OCH3 |
H3PO4 |
|
|
|
|
|||||
H3CO |
|
|
[NBu3(C6 H13)][NTf2] |
||||||||
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
4 hours, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
75-80 °C |
H3CO |
|
|
||
Scheme 5.1-57: The cyclization of 3,4-dimeth- |
|
|
|||||||||
H3CO |
|||||||||||
oxyphenylmethanol in an ionic liquid. |
|||||||||||
5.1.2.3Friedel–Crafts acylation reactions
OCH3
83-89 %
 OCH3
OCH3
OCH3
Friedel–Crafts acylation reactions usually involve the interaction of an aromatic compound with an acyl halide or anhydride in the presence of a catalyst, to form a carbon–carbon bond [74, 75]. As the product of an acylation reaction is less reactive than its starting material, monoacylation usually occurs. The “catalyst” in the reaction is not a true catalyst, as it is often (but not always) required in stoichiometric quantities. For Friedel–Crafts acylation reactions in chloroaluminate(III) ionic liquids or molten salts, the ketone product of an acylation reaction forms a strong complex with the ionic liquid, and separation of the product from the ionic liquid can be extremely difficult. The products are usually isolated by quenching the ionic liquid in water. Current research is moving towards finding genuine catalysts for this reaction, some of which are described in this section.
The first example of a Friedel–Crafts acylation reaction in a molten salt was carried out by Raudnitz and Laube [90]. It involved the reaction between phthalic anhydride and hydroquinone at 200 °C in NaCl/AlCl3 (X(AlCl3) = 0.69) (Scheme 5.1-58).
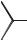
Scholl and co-workers [91] performed the acylation of 1-benzoylpyrene with 4- methylbenzoyl chloride in a NaCl/AlCl3 (X(AlCl3) = 0.69) molten salt (110–120 °C). This gave 1-benzoyl-6-(4-methylbenzoyl)-pyrene as the major product (Scheme 5.1-59).
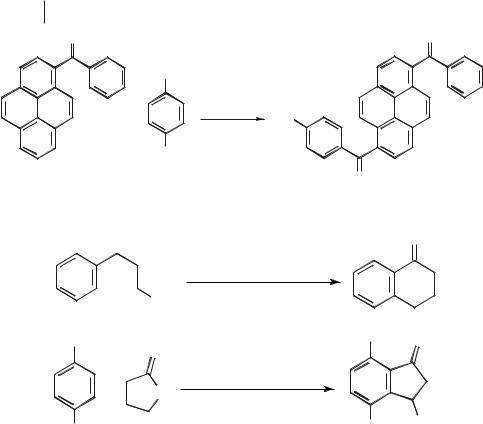
Bruce et al. carried out the cyclization of 4-phenylbutyric acid to tetralone in NaCl/AlCl3 (X(AlCl3) = 0.68) at 180–200 °C [92]. The reaction between valerolactone and hydroquinone to give 3-methyl-4,7-dihydroxyindanone was also performed by Bruce, using the same ionic liquid and reaction conditions. These are shown in Scheme 5.1-60.
O |
OH |
O |
OH |
O |
NaCl-AlCl3 (X = 0.69) |
|
|
+ |
°C, 10 min |
|
|
|
200 |
|
|
O |
OH |
O |
OH |
|
|||
Scheme 5.1-58: The reaction between phthalic anhydride and hydroquinone in NaCl/AlCl3 (X(AlCl3) = 0.69).
204 Martyn Earle |
|
|
|
O |
|
|
O |
|
COCl |
|
|
|
|
NaCl-AlCl3 |
|
|
+ |
(X = 0.69) |
|
|
H3C |
|
|
|
|
110-120 °C |
|
|
CH3 |
|
|
|
|
O |
|
Scheme 5.1-59: The acylation of 1-benzoylpyrene in NaCl/AlCl3 (X(AlCl3) = 0.69). |
|||
|
|
|
O |
|
|
NaCl-AlCl3 (X = 0.68) |
|
|
CO2H |
180-200 °C, 2 min |
|
|
|
|
|
OH |
|
OH |
O |
O |
|
||
|
|
|
|
+ |
NaCl-AlCl3 (X = 0.68) |
|
|
O |
180-200 °C, 2 min |
|
|
|
|
||
OH |
|
OH |
CH3 |
|
|
|
|
Scheme 5.1-60: The use of NaCl/AlCl3 (X(AlCl3) = 0.68) in the formation of cyclic ketones.
The Fries rearrangement can be viewed as a type of Friedel–Crafts acylation reaction. Two examples of this reaction are given in Scheme 5.1-61. The first is the rearrangement of 4,4’-diacetoxybiphenyl to 4,4’-dihydroxy-3,3’-diacetoxybiphenyl in a NaCl/AlCl3 (X(AlCl3) = 0.69) molten salt [93]. The second example is the rearrangement of phenyl 3-chloropropionate to 2’-hydroxy-3-chloropropiophenone, followed by cyclization to an indanone [94].
One of the problems with the NaCl/AlCl3 molten salts is their high melting points and corresponding high reaction temperatures. The high reaction temperatures tend to cause side reactions and decomposition of the products of the reaction. Hence, a number of reactions have been carried out under milder conditions, in room-temperature ionic liquids. The first example of a Friedel–Crafts acylation in such an ionic liquid was performed by Wilkes and co-workers [66, 79] (Scheme 5.1-62). The rate of the acetylation reaction was found to be dependent on the concentration of the [Al2Cl7]– ion, suggesting that this ion was acting as the Lewis acid in the reaction. Wilkes went on to provide evidence that the acylating agent is the acetylium ion [H3CCO]+ [66, 79].

5.1 Stoichiometric Organic Reactions and Acid-Catalyzed Reactions in Ionic Liquids 205 |
|
||||
O |
|
|
|
|
|
O |
R |
|
OH |
O |
|
|
|
|
|
R |
|
|
NaCl-AlCl3 (X = 0.69) |
|
|
|
|
|
200 °C, 2 min |
|
|
|
|
|
|
|
|
R |
|
O |
R |
|
OH |
O |
|
O |
|
|
|
|
|
|
chloro- |
OH O |
|
OH |
O |
O |
aluminate(III) |
|
|||
|
|
|
|||
molten salt |
|
|
|
|
|
|
|
Cl |
|
|
|
O |
Cl 160 °C |
|
|
|
|
|
|
|
|
||
Scheme 5.1-61: The Fries rearrangement in chloroaluminate(III) molten salts.
|
|
O |
|
|
|
|
|
O |
||||||
+ |
H3C |
|
|
|
Cl |
[EMIM]-AlCl3 (X = 0.67) |
|
|
|
|
||||
|
|
|
|
|
||||||||||
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 5.1-62: The acetylation of benzene in a room-temperature ionic liquid.
A number of commercially important fragrance molecules have been synthesized by Friedel–Crafts acylation reactions in these ionic liquids. Traseolide® (5-acetyl- 1,1,2,6-tetramethyl-3-isopropylindane) (Scheme 5.1-63) has been made in high yield in the ionic liquid [EMIM]Cl/AlCl3 (X(AlCl3) = 0.67) [95].
For the acylation of naphthalene, the ionic liquid gives the highest reported selectivity for the 1-position [95]. The acetylation of anthracene at 0 °C was found to be a reversible reaction. The initial product of the reaction between acetyl chloride (1.1 equivalents) and anthracene is 9-acetylanthracene, formed in 70 % yield in less than 5 minutes. The 9-acetylanthracene was then found to undergo diacetylation reactions, giving the 1,5- and 1,8-diacetylanthracenes and anthracene after 24 hours (Scheme 5.1-64).
