
Emerging Tools for Single-Cell Analysis
.pdf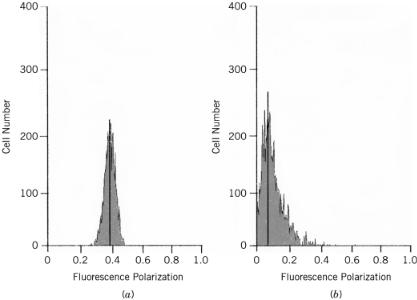
Application of Flow Cytometry for Monitoring Antigen Processing and Presentation |
211 |
F i g . 9.10. Fluorescence polarization histograms of Immunobrite III beads or J774 macrophages preincubated with fluoresceinated peptides. (a) The fluorescence polarization of Immunobrite beads was monitored. (b) Cells were incubated with fluoresceinated peptides and fluorescence polarization was monitored.
ter was calibrated accurately, the cells were incubated with FITC10BSA, and fluorescence polarization was monitored. Figure 9.9 represents various fluorescence intensity histograms depicting both the vertical and horizontal fluorescence components of the cells and illustrates several important points concerning the measurements. First, in terms of the processing of the probe, the cells were relatively homogeneous, producing Gaussian distributions that facilitated data analysis (Fig. 9.9). However, as evident from the data, within the population there was a wide range of intensities, which indicated that the cells were at slightly different stages of processing (Fig. 9.9). For example, the low fluorescence intensities could be attributed to cells that had only recently begun to degrade the probe while the higher fluorescence intensities could be the result of cells in which extensive proteolytic degradation had already occurred (Fig. 9.9). As described in the Introduction, several different events occur as proteins proceed through the endocytic compartment. Since the probe employed in these studies was sensitive to each of these steps, it was not unexpected to see variability in the intensity data (Fig. 9.9). In short, these results simply reflected the wide variety of events that occurred within the cells rather than improper calibration of the instrument (Fig. 9.9, 9.10). Furthermore, to ensure that the results were accurate, no less than 90% of the gated population was represented in each calculation, and both the horizontal and vertical gains were adjusted throughout the experiment to ensure an accurate representation of the dynamic population.

212 |
Application of Fluorescence Lifetime and Two-Photon Fluorescence Cytometry |
Results of fluorescence polarization measurements of macrophage cells incubated with FITC10BSA for various periods of time are depicted in Figure 9.11. A steady increase in fluorescence polarization was observed for the first 100 min of the experiment (Fig. 9.11). The fluorescence polarization then remained constant for the next 100 min, and finally, a decrease in fluorescence polarization was observed after 200 min (Fig. 9.11). Decreased fluorescence polarization at 200 min was the result of proteolytic cleavage of FITC10BSA and generation of relatively small fluoresceinated peptides; yet as indicated in fluorescence polarization histograms, the degree of degradation varied slightly from cell to cell (Fig. 9.11; Weaver et al., 1996, 1997). Increased fluorescence polarization could be caused by several factors, including: (1) an increase in the viscosity of the endocytic compartment, (2) a decrease in the pH of the local environment of the fluorophore, and (3) binding by a macromolecule. However, control experiments demonstrated that these data reflected the kinetics of endosomal transport by macrophages. To test this hypothesis more directly, the fluorescence polarization of cells treated with the weak base ammonium chloride was monitored as well (Poole and Ohkuma, 1981). When the fluorescence polarization of the cells was monitored, the increase in fluorescence polarization was not observed; instead the fluorescence polarization decreased, reaching a plateau at ~0.10. Extensive studies by Ohkuma and Poole (1978) have shown that upon addition of ammo-
F i g . 9.11. Cell-by-cell analysis of the fluorescence polarization of macrophage cells incubated with FITC10BSA. Cells incubated with 10 g/ml FITC10BSA for various periods of time were analyzed on a cell-by-cell basis and plotted as a function of fluorescence polarization.
Application of Flow Cytometry for Monitoring Antigen Processing and Presentation |
213 |
nium chloride the endocytic environment reached a pH of 6.5. Therefore, a polarization value of ~0.10 would be expected. In short, the flow cytometry polarization analysis provided further evidence that FITC10BSA was localized to the endocytic environment as well as provided a method to observe endocytic transport.
Fluorescence Lifetime Measurements
The recent development of a flow cytometer capable of measuring fluorescence lifetime at the University of Illinois Flow Cytometry Facility has provided an important tool for dissecting the intracellular microenvironment of macrophage. Specifically, fluorescence lifetime measurements offer a powerful tool for uniting both structural and functional details of biological processes. In the case of the present system, fluorescence lifetime measurements proved invaluable for measuring both intracellular trafficking and degradation of the antigenic probe. However, in the application of fluorescence lifetime measurements to antigen-processing studies, two important factors will affect data. First, as indicated in Figure 9.3, fluorescein’s fluorescence lifetime is exquisitely sensitive to pH. Therefore, as the antigenic probe migrates through the endocytic system, a decrease in fluorescence lifetime will be detected. Second, when the protein carrier is in the globular form, fluorescein’s fluorescence lifetime is quenched due to the close spatial proximity and orientation of neighboring fluorescein molecules (Fig. 9.4). Therefore, as the spatial constraint is alleviated via proteolytic cleavage of the protein carrier, an increase in fluorescence lifetime will also be detected.
To perform fluorescence lifetime experiments, several standards were used to calibrate the instrument, including acridine orange, ethidium bromide, and fluorescein- 5-isothiocyanate with corresponding fluorescence lifetime values of 1.7, 1.6, and 4.0 ns, respectively. As indicated in Table 1, several trends were observed in the lifetime experiments. First, slight variations were observed when comparing measurements of fluorescein-derivatized probes using different reference standards. For example, when cells were incubated with FITC20BSA for 2 h, fluorescence lifetime values of 2.4, 2.2, and 2.1 ns were observed using acridine orange, ethidium bromide, and fluorescein reference standards, respectively (Table 1). In the case of FITC10BSA, values of 2.6 2.4, and 2.1 ns were observed for acridine orange, ethidium bromide, and FITC reference standards, respectively (Table 1). Therefore, selection of the appropriate standard is imperative. Second, lifetime experiments verified previous fluorescence intensity and polarization experiments that suggested the probe localized to acidic compartments within the cell since low fluorescence lifetime values were observed even after 24 h (Table 1). More importantly, as time progressed, increased fluorescence lifetime values were observed in the case of fluorescein-derivatized BSA, indicative of degradation within the endocytic system (Table 1). However, when flu- orescein-derivatized poly-D-lysine was incubated with the cells, increased fluorescence lifetime values were not detected, which was expected since poly-D-lysine is nondegradable (Table 1). Therefore, flow cytometry fluorescence lifetime measurements further validated the use of FITC–BSA as a tool for monitoring the endocytic processing of proteins.

214 |
Application of Fluorescence Lifetime and Two-Photon Fluorescence Cytometry |
T A B L E 1. Fluorescence Lifetime Flow Cytometry Measurements of Fluores- cein-Derivatized Probes Following Endocytosis and Processing by Murine Macrophage Cell Line J774
Antigenic Probe |
Incubation Time (min) |
Lifetime (ns) |
|
|
|
Acridine Orange Reference Standard (1.7 ns) |
|
|
FITC10BSA |
60 |
2.6 |
FITC20BSA |
5 |
1/2 |
FITC20BSA |
10 |
1.2 |
FITC20BSA |
60 |
2.6 |
FITC20BSA |
120 |
2.4 |
FITC20BSA |
240 |
2.1 |
FITC5PDL |
60 |
1.9 |
FITC5PDL |
120 |
1.9 |
FITC standard |
|
4.0 |
Ethidium bromide standard |
|
1.8 |
|
FITC Reference Standard (4.0 ns) |
|
FITC10BSA |
60 |
2.1 |
FITC20BSA |
60 |
2.1 |
FITC20BSA |
120 |
2.2 |
FITC20BSA |
240 |
2.2 |
FITC5PDL |
60 |
1.6 |
FITC5PDL |
120 |
1.6 |
Acridine orange standard |
|
1.4 |
Ethidium bromide standard |
|
1.5 |
Ethidium Bromide Reference Standard (1.6 ns) |
|
|
FITC10BSA |
60 |
2.4 |
FITC20BSA |
5 |
1.3 |
FITC20BSA |
10 |
1.4 |
FITC20BSA |
60 |
2.4 |
FITC20BSA |
120 |
2.2 |
FITC20BSA |
240 |
2.4 |
FITC5PDL |
60 |
1.9 |
FITC5PDL |
120 |
1.9 |
FITC standard |
|
4.1 |
Acridine orange standard |
|
1.6 |
|
|
|
Note: PDL, poly-D-lysine. Subscripts designate the moles of fluorophore per mole of protein carrier. Reference standards represent the compound used to calibrate the instrument prior to performing experiments.

Conclusions |
215 |
When comparing fluorescence lifetime values obtained through flow cytometry and two-photon fluorescence imaging, similar values were observed (French et al. 1997). For example, following a 24-h incubation, a fluorescence lifetime of 2.4 ns was detected using flow cytometry while a value of 2.3 ns was detected using twophoton fluorescence microscopy (French et al., 1997). In the case of FITC–poly-D-ly- sine, values of 1.2 and 1.3 ns were observed for flow cytometry and two-photon fluorescence microscopy, respectively (French et al., 1997). The similarity in data validates the accuracy of flow cytometry fluorescence lifetime measurements. One obvious advantage of using two-photon fluorescence microscopy is that the three-dimensional imaging capabilities afforded by two-photon excitation can be used to monitor specific regions within the cells (French et al., 1997). However, in certain experimental systems, flow cytometry may offer an alternative since flow cytometry provides information on populations of cells in a short period of time, although total cell-associated fluorescence is detected.
CONCLUSIONS
The complexities of antigen processing and presentation require the development of novel technologies capable of providing new insights into these cellular processes. Therefore, the goal of this chapter is to emphasize the significance of a novel antigenic probe and its application to fluorescence microscopy and flow cytometry (Voss et al., 1996; Weaver et al., 1996). The fluorescent properties of FITC-derivatized BSA provide for analysis of intracellular events via multiple parameters, including fluorescence lifetime, intensity, and polarization. Integration of these fluorescent techniques ensures that the inherent biases of individual measurements do not influence experiments. For example, fluorescence intensity and polarization are biased toward the most intense signals within a cell. Therefore, signals derived from small or extensively degraded peptides will predominate results while signals derived from partially degraded peptides will be masked. However, in the case of fluorescence lifetime, all signals are registered independent of intensity, resulting in an average, albeit a weighted one. Thus, to determine the kinetics of intracellular pathways, all three parameters are required to establish an accurate representation of events. Moreover, this probe has also been used in combination with invasive techniques such as electron microscopy and subcellular fractionation, and the ability of each of these methodologies, including flow cytometry, two-photon fluorescence microscopy, and subcellular fractionation, to complement one another represents a powerful system for the elucidation of various intracellular phenomena (Weaver et al., 1998a,b). To illustrate this, a model for the processing and presentation of MHC II–peptide complexes is presented here. Moreover, this model is based solely on data obtained by combining the novel fluorescent antigen with the techniques described above.
The antigenic protein entered the cell through multiple processes including pinocytosis and receptor-mediated endocytosis (Fig. 9.12; Cherukuri et al., 1997a; Cherukuri and Voss, 1998). Upon entering the cell, the antigen probe was internalized into an acidic environment, and within 5 min, the protein localized to early endocytic

216 |
Application of Fluorescence Lifetime and Two-Photon Fluorescence Cytometry |
organelles (Weaver and Voss, 1998b). More importantly, degradation of the protein was also evident by 5 min. As time progressed, the protein was detected in late endosomal/lysosomal organelles by 10 min (Weaver and Voss, 1998b). Flow cytometry experiments suggested that thiol and aspartyl proteases were the primary enzymes involved in the cleavage of the probe (Weaver and Voss, 1998b). MHC II–peptide loading of antigenic fluoresceinated peptides was observed by 15 min in transferrin receptor–positive, LAMP-1–positive, and cathepsin D–positive organelles, whereas loading of unlabeled BSA peptides occurred in transferrin receptor–negative organelles (Weaver and Voss, 1998b). In addition, MHC II–fluoresceinated peptide complexes utilized transferrin receptor–positive organelles for transport to the plasma membrane at 30 min (Weaver and Voss, 1998b). Therefore, macrophage cells utilized
F i g . 9.12. Kinetic model for MHC II–peptide loading and surface expression in murine macrophages. The antigenic protein enters the cell through receptor-mediated endocytosis or pinocytosis. Upon entering the cell, the antigenic protein encounters an acidic environment at 5 min. Also at 5 min, proteolytic degradation of the protein begins, and by 10 min, the accumulation of antigenic peptides within the late endosomal/lysosomal environment is observed. Exocytosis of fluoresceinated peptides begins at 15 min, but more importantly, MHC II–peptide loading of fluoresceinated peptides occurs in transferrin receptor–pos- itive or –negative late endosomal organelles. Finally, MHC II–fluoresceinated peptide complexes migrate to the plasma membrane via a retrograde pathway for surface expression at 30 min.

References |
217 |
a retrograde pathway through the endocytic system to transport MHC II–peptide complexes to the cell surface (Weaver and Voss, 1998b; Fig. 9.12).
This technique is not restricted to proteolytic processing by macrophages. Using various fluorescent probes and carrier proteins, the microenvironment of a wide variety of macromolecules could be monitored noninvasively in viable cells, facilitating kinetic analysis and providing insight into numerous cellular processes. For example, several of the downstream effector molecules responsible for programmed cell death were recently identified (Boldin et al., 1996; Muzio et al., 1996). Termed caspases, these enzymes are believed to act as a signal cascade to initiate apoptosis (Wang and Lenardo, 1997). With the recent development of membrane-permeable caspase substrates, the construction of fluorescent substrates similar to FITC–BSA is possible and the development of a fluorescent system to monitor the kinetics of caspase activity in live cells is also feasible. As indicated in the macrophage system, directly measuring the kinetics of intracellular processes in live cells can offer several advantages over conventional techniques and provide new insights into the mechanisms responsible for those kinetics.
REFERENCES
Amigorena S, Drake J, Webster P, Mellman I (1994): Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature 369:113–120.
Benjamin DC, Berzofsky JA, East IJ, Gurd FRN, Hannum C, Leach SJ, Margoliash E, Michael JG, Miller A, Prager EM, Secarz EE, Smith-Gill SJ, Todd PE, Wilson AC (1984): The antigenic structure of proteins: reappraisal. Annu Rev Immunol 2:67–102.
Boldin MP, Goncharov TM, Goltsev YV, Wallach D (1996): Involvement of MACH, a novel MORT1/FADD-interacting protease, in FAS/APO-1 and TNF receptor induced cell death. Cell 85:803–815.
Bowser R, Murphy RF (1990): Kinetics of hydrolysis of endoctyosed substrates by mammalian cultured cells: early introduction of lysosomal enzymes into the endocytic pathway. J Cell Physiol 143:110–117.
Cherukuri A, Voss EW, Jr. (1998): Ligand binding specificity of a macrophage surface receptor utilized by the fluorescein hapten for uptake into the endocytic pathway. Mol Immunol 35:115–125.
Cherukuri A, Nelson J, Voss EW, Jr (1998): Biochemical purification and partial characterization of a macrophage surface receptor possessing specificity for small aromatic moieties including fluorescein. J Mol Recognition 12:94–102.
Cherukuri A, Durack G, Voss EW, Jr (1997a): Evidence for hapten recognition in receptor-mediated intracellular uptake of a hapten-protein conjugate by murine macrophage. Mol Immunol 34:21–32.
Cherukuri A, Frye J, French T, Durack G, Voss E.W., Jr. (1997b): FITC-poly-D-lysine conjugates as fluorescent probes to quantify hapten-specific macrophage receptor binding and uptake kinetics. Cytometry 31:110–124.
Cresswell P (1994): Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol 12:259–293.
Diment S (1993): Proteolytic processing for MHC class II antigen presentation. Ciencia Cultura 45:305–312.
Fineschi B, Miller J (1997): Endosomal processing and antigen processing. Trends Biol Sci 22:377–382.
French T, So PTC, Weaver DJ, Jr, Coelho-Sampaio T, Gratton E, Voss EW, Jr, Carrero J (1997): Two-pho- ton fluorescence lifetime imaging microscopy of macrophage-mediated antigen processing. J Microsc 185:339–353.
218 |
Application of Fluorescence Lifetime and Two-Photon Fluorescence Cytometry |
Germain RN, Marguiles DH (1993): The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol 11:403–450.
Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ (1997): Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol 139:639–649.
Kranz DM, Voss EW, Jr (1981): Partial elucidation of an anti-napter repertoire in BALB/c mica: comparative characterization of several monoclonal anti-fluoresay antibodies. Mol Immunol 18:889–898.
Lanzavecchia A (1990): Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol 8:773–793.
Lakowicz JR (1983): Principles of Fluorescence Spectroscopy. New York: Plenum Press.
Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R (1996): FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (FAS/APO-1) death-inducing signal complex. Cell 85:817–827.
Ohkuma S, Poole B (1978): Fluorescence probe measurement of intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75:3327–3331.
Pieters J (1997): MHC class II restricted antigen presentation. Curr Opin Immunol 9:89–96.
Poole B, Ohsuma S (1981): Effect of weak bases on the intralysosonal pH in mouse peritoneal macrophages. J Cell Biol 90:665–669.
Qui Y, Xu X, Wandinger-Ness A, Dalke DP, Pierce SK (1994): Separation of subcellular compartments containing distinct forms of MHC class II. J Cell Biol 125:595–605.
Rothe G, Valet G (1993a): Measurement of neutrophil elastase activity with (N-benzyloxycarbonyl-Ala- Ala)2-Rhodamine 110). In Robinson JP (ed). Handbook of Flow Cytometry Methods. New York: Wiley, pp 200–201.
Rothe G, Valet G (1993b): Measurement of mononuclear phagocyte cathepsin B/L activity with (N- benzyloxycarbonyl-Arg-Arg)2-Rhodamine 110). In Robinson JP (ed). Handbook of Flow Cytometry Methods. New York: Wiley, pp 202–203.
Rothe G, Oser A, Assfalg-Machleidt I, Machleidt W, Mangel WF, Valet G (1990): Cathepsin B activity measured with (Z-Phe-Arg)2-Rhodamine 110 as a new flow cytometric marker of monocyte/macrophage activation. Cytometry Suppl. 4:77.
Sklar LA, Finney DA (1982): Analysis of ligand-receptor interactions with the fluorescence activated cell sorter. Cytometry 3:161–165.
Sklar LA, Finney DA, Oades ZG, Jesaitis AJ, Painter RG, Cochrane CG (1984): The dynamics of ligandreceptor interactions. Real-time analyses of association, dissociation, and internalization of an N- formyl peptide and its receptors on the human neutrophil. J Biol Chem 259:5661–5669.
Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J (1994):Isolation and characterization of the intracellular MHC class II compartment. Nature 369:120–126.
Voss EW, Jr. (1984): Fluorescein Hapten: An Immunological Probe. Boca Raton, FL: CRC Press.
Voss EW, Jr (1990): Anti-fluorescein antibodies as structure function models to examine fundamental immunochemical and spectroscopic principles. Comments Mol Cell Biophys 6:197–221.
Voss EW, Jr, Workman CJ, Mummert ME (1996): Detection of protease activity using a fluorescentenhancement globular substrate. BioTechniques 20:286–291.
Ward ES, Qadri O (1997): Biophysical and structural studies of TCRs and ligands: implication for T cell signaling. Curr Opin Immunol 9:97–106.
Watts C (1997): Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol 15:821–850.
Wang J, Lenardo MJ (1997): Molecules involved in cell death and tolerance. Curr Opin Immunol 9:818–825.
References |
219 |
Weaver DJ, Jr, Voss EW, Jr (1998a): Analysis of rates of receptor-mediated endocytosis and exocytosis of a fluorescent hapten-protein conjugate in murine macrophage: implications for antigen processing. Biol Cell 90:169–181.
Weaver DJ, Jr, Voss EW, Jr (1998b): A novel macrophage receptor enhances MHC II-peptide loading and surface expression of a hapten-protein conjugate. Biol Cell. 90:427–438.
Weaver DJ, Jr, Voss EW, Jr (1999): Kinetics and intracellular pathways required for major histocompatibility complex II-peptide loading and surface expression of a fluorescent hapten-protein conjugate in murine macrophage. Immunology 96:557–568.
Weaver DJ, Jr, Durack G, Voss EW, Jr (1997): Analysis of the intracellular processing of proteins: application of fluorescence polarization and a novel fluorescent probe. Cytometry 28:25–35.
Weaver DJ, Jr, Cherukuri A, Carrero J, Coehlo-Sampaio T, Durack G, Voss EW, Jr (1996): Macrophagemediated processing of an exogenous antigenic fluorescent probe: Time-dependent elucidation of the processing pathway. Biol Cell 87:95–104.
Weber G (1952): Polarization of the fluorescence of macromolecules. I. Theory and experimental methods. Biochem J 51:145–155.
Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J (1996): Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol 135:611–622.
Xu X, Song W, Cho H, Qiu Y, Pierce SK (1995): Intracellular transport of invariant chain MHC II complexes to the peptide loading compartment. J Immunol 155:2984.–2992.

Emerging Tools for Single-Cell Analysis: Advances in Optical Measurement Technologies
Edited by Gary Durack, J. Paul Robinson Copyright © 2000 Wiley-Liss, Inc.
ISBNs: 0-471-31575-3 (Hardback); 0-471-22484-7 (Electronic)
10
Probing Deep-Tissue Structures by Two-Photon Fluorescence Microscopy
Chen-Yuan Dong, Ki Hean Kim, Christof Buehler, Lily Hsu, Hyun Kim, and Peter T. C. So
Massachusetts Institute of Technology, Cambridge, Massachusetts
Barry R. Masters
University of Bern, Bern, Switzerland
Enrico Gratton
University of Illinois at Urbana-Champaign, Urbana, Illinois
Irene E. Kochevar
Massachusetts General Hospital, Boston, Massachusetts
INTRODUCTION
Two-photon fluorescence microscopy is increasingly becoming an important part of modern optical techniques. Excellent axial depth discrimination, limitation of photodamage to focal volume, good depth penetration, and more efficient fluorescence detection are some of the key advantages of two-photon microscopy over conventional techniques. This chapter begins with an overview of two-photon microscopy relevant to applications in deep-tissue imaging. Basic principles, deep-tissue models, and
221
