
Multiple Bonds Between Metal Atoms / 12-Rhodium Compounds
.pdf
|
|
|
|
|
|
|
|
|
Compound |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
r (Rh–Rh)a (Å) |
r (Rh–Lax)b (Å) |
Donor |
ref. |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
atom(s) |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
{Rh |
[O |
C(CH |
) |
OC |
H OC(CH |
) |
CO |
][O |
CC |
6 |
H |
(OH) |
CO |
]} |
·11EtOH·2CH |
3 |
CO |
C |
H · |
2.383(1) |
2.238c,ccc |
O |
114 |
||||||||||||||||||
2 |
2 |
3 |
2 |
6 |
4 |
3 |
2 |
2 |
2 |
|
|
2 |
|
|
|
|
|
|
2 |
|
|
|
2 |
4 |
|
|
|
|
|
|
|
|
2 |
2 |
5 |
|
|
|
|
||
2H2Oxxx |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.382(1) |
2.305c,ll |
|
|
|
{Rh |
[O |
C(CH |
) |
OC |
H OC(CH |
) |
CO |
][O |
CC |
10 |
H |
6 |
CO |
|
]} |
|
·16CH |
3 |
OH·H |
Oxxx |
|
|
|
|
|
|
2.383(1) |
2.280c,ccc |
O |
114 |
|||||||||||
2 |
2 |
3 |
2 |
6 |
4 |
3 |
2 |
2 |
2 |
|
|
|
|
2 |
|
4 |
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.379(1) |
2.294c,ccc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.260c,ccc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.264c,ccc |
|
|
{Rh |
[O |
C(CH |
) |
OC |
H OC(CH |
) |
CO |
][O |
CC |
6 |
Cl |
CO |
|
]} |
·10EtOH·2CH |
|
CO C |
2 |
H xxx |
|
2.389(1) |
2.309c,ccc |
O |
114 |
|||||||||||||||||
2 |
2 |
3 |
2 |
6 |
4 |
3 |
2 |
2 |
2 |
|
|
4 |
|
|
2 |
|
|
4 |
|
|
|
|
|
|
|
3 |
2 |
|
|
5 |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.388(1) |
2.299c,ccc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.276c,ccc |
|
|
{Rh |
[O |
C(CH |
) |
OC |
H OC(CH |
) |
CO |
][O |
CC |
6 |
(CH |
) |
CO |
|
]} |
·10CH OH·12H |
2 |
Oxxx |
|
|
2.373(1) |
2.285c,ccc |
O |
114 |
|||||||||||||||||
2 |
2 |
3 |
2 |
6 |
4 |
3 |
2 |
2 |
2 |
|
|
|
|
|
3 |
4 |
|
|
|
|
2 |
4 |
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.380(1) |
2.261c,ccc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.312c,ccc |
|
|
aDistances are given with up to 3 decimal digits.
bIn some cases the average Rh–L bond lengths are quoted. In these instances
|
the |
estimated deviation, which is |
given |
in square brackets, |
is calculated |
as |
|
[ ] = [Ψn¨i2/n(n < 1)]1/2, in which ¨i |
is the deviation of the ith of n values from the |
||||
|
arithmetic mean of the set. |
|
|
|
|
|
c |
Esds not reported. |
|
|
|
|
|
|
|
|
|
|
||
d |
The |
longer of these two distances is |
Rh–O (H2O), the |
shorter one |
is |
|
|
||||||
Rh–O (HCO2<).
eCrystal structure determined by X-ray powder diffraction.
fRh–O distance to the carboxylate bridge of neighboring Rh24+ unit.
gCarboxylate bridge of neighboring Rh24+ unit.
hThe compound contains ax acetate groups.
iThe longer of these two distances is Rh–N (NO2), the shorter one is Rh–N (NO).
jLow resolution structure (4 Å) due to poor quality of crystals.
kDifferent crystalline form from that in ref. 138.
lDistance to the pyridine coordination site.
m |
Distance to the amine coordination site. |
|
nThe crystal contains two kinds of dirhodium units: one with the ax sites occupied by pyridine nitrogen atoms and one with the ax sites occupied by amino nitrogen
atoms.
oNitrogen atom of the pyridine ring.
pThe compound crystallizes in the space group C2/c.
q |
– |
|
The compound crystallizes in the P1 space group. |
||
|
rRh–N distance to pyrimidine ring nitrogen.
sRh–N distance to the aminomethyl substituent nitrogen.
tOnly the unit cell has been determined.
uRh–P distance.
vAverage distance.
wBinding takes place via a pendant NH2 group of the acridine ligand.
xCentrosymmetric ‘dimer of dimers’.
yDistance to O of ax acetone molecule.
zRh–S distance.
aaRh–O distance.
bbThere are three Rh24+ units with only O atoms at the ends, two with only S atoms and two with both O and S atoms.
ccRh–O distance to the DMSO oxygen atom.
Dunbar andChifotides |
|
Compounds Rhodium |
|
485 |
|
ddArray of six rhodium atoms linked into infinite chains {[Rh2(µ-O2CCF3)2(CO)4][Rh2(µ- O2CCF3)4][Rh2(µ-O2CCF3)2(CO)4]} .
eeAxial distance of Rh2(µ-O2CCF3)4 Rh atom to Rh atom of neighboring Rh2(µ-O2CCF3)2(CO)4 unit.
ffRh(I)···Rh(I) distance within the Rh2(µ-O2CCF3)2(CO)4 unit.
ggRh···Rh distance between two adjacent Rh2(µ-O2CCF3)2(CO)4 moieties.
hhThis Rh–Rh distance is encountered in the Rh24+ unit with two bidentate and two monodentate CF3CO2 ligands.
iiThe Tempol ligand is bound through its hydroxyl group.
jjDistance to alkyne carbon atom.
kkDistance to arene carbon atom.
llDistance to ax Ocarbonyl.
mmC12H8: acenaphthylene.
nnC12H10: acenaphthene.
ooThe second set of Rh···C distances is the same.
ppC14H10: anthracene.
qqC14H10: phenanthrene.
rrC16H10: pyrene.
ssAll four Rh···C distances are the same.
ttC16H10: fluoranthene.
uuδ1-coordination.
vvC18H12: 1,2-benzanthracene.
wwC18H12: triphenylene.
xxC18H12: chrysene.
yyC20H10: corannulene.
zzC30H12: hemibuckminsterfullerene.
aaaAverage value of two Rh–C distances for each exo-coordinated rhodium center; δ2-coordination mode.
bbbRh–C distance of endo-bound rhodium atom; δ1-coordination mode.
cccDistance to ax Oalcohol.
dddDistance determined by EXAFS.
eeeNo distance reported.
fffDistance in butyrate adduct.
gggDistance in p-hydroxybenzoate adduct.
hhhDistance to carbon of double bond.
iiiSame Rh–O distance to EtOH and H2O molecules.
jjjMixed Rh:Cu complex (88:12 ± 3%).
kkkNo coordinates available.
lllTetracarboxylate compound with no ax ligands.
mmmThe value refers to the ax water molecule.
nnnThere is a toluene molecule oriented in an δ2 fashion towards the other ax position at an average distance of 2.80 Å.
oooDistance to the carbonyl group of an ax TiPBH molecule.
pppA toluene molecule is oriented in a δ2 fashion towards the free ax position of each subunit at an average distance of 2.70 Å.
qqqDistance to N atom of pyridyl group.
rrrA benzene molecule is present at the open ax end of each dirhodium unit.
sssMetal-/ interactions with the phenyl groups of the ligand.
tttDistance to O of OH group.
uuuDistance to carbon atom of the pyridyl ring.
vvvDistance to carbon atom of the benzene molecule that occupies one ax site.
wwwWeak interactions with the aromatic carbons of the aniline ring axially bound to the flanking dirhodium unit.
xxxMolecular square.
yyyRange of distances.
zzzH2L: 2,7-di-But-9,9-dimethyl-4,5-xanthenedicarboxylic acid.
aaaaMacrocyclic dimer.
bbbbTHF oxygen atom.
|
486 |
|
12 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |

Rhodium Compounds 487
Chifotides and Dunbar
The most commonly encountered Rh2(O2CR)4L2 compounds in the series adopt the discrete structure 12.11 and consist of the dirhodium core with essentially D4h symmetry and almost invariably two ax ligands L that typically are identical. Exceptions include Rh2(O2CC6H4- 2-OH)4(EtOH)(H2O),71 Rh2(O2CCH3)4(NO)(NO2),134,216,217 Rh2(TiPB)4(H2O)(δ2-C6H5CH3),75 Na[Rh2(µ-O2CH)4(µ3-δ1:δ1:δ1-O2CH)(H2O)],235 the dimeric adduct [Rh2(O2CCF3)4(Me2CO)]2- (µ2-δ2:δ2-C4I2)59 and the rare tetracarboxylate compound Rh2(TiPB)4(NCCH3), which has only one ax ligand.75 The structural features of the monohydrate Rh2(O2CH)4(H2O),28,302 obtained from a formic acid solution containing small quantities of water, and the adducts Rh2(O2CCH3)4L (L = DMSO, SEt2), which were prepared as bulk materials by thermal decomposition of the corresponding bis-adducts,43 have not been confirmed.
Another dirhodium tetracarboxylate group comprises 1:1 Rh2(O2CR)4L adducts arranged in polymeric infinite structures (Fig. 12.2a). In general, the ax ligands encountered in this group contain at least two binding sites, but there are a few exceptions such as those of the pyramidal complex {[Rh2(O2CCF3)4]3CH3Si(C5H4N)3(δ1-C6H6)3} with three donor atoms of the pyridyl groups bridging three dirhodium units,303 a few cases where the ligand exhibits tetradentate behavior (for Rh2(O2CR)4L, with R = CF3 and L = TCNE (Fig. 12.3),200 R = CF3 and L = TCNQ,201 R = CH3 and L = Co(CN)63-,304 and R = CF3 and L = (C6H5)2Si(C5H4N)2 with the ligand binding via two N-donor atoms and two phenyl groups303), the adducts of the tetrakis(trifluoroacetate) with 1,4-diiodo-1,3-butadiyne,59 and tri-, tetraor multidentate aromatic hydrocarbons,57,58 as well as the case of the unusual supramolecular assembly of {[Rh2(O2CCF3)4]3[µ5-(HO)C(C5H4N)3](δ1-C6H6)}.303 There are a few examples, however, wherein a single donor atom of the ligand is engaged in bridging two Rh24+ units, leading to 1-D chain structures, e.g., [Rh2(O2CCF3)4(µ-DMSO-O)] ,51 [Rh2(O2CCF3)4(THF)] (Fig. 12.4),49 [Rh2(O2CCF3)4(Me2SeO)] 238 and [Rh2(O2CCF3)4(Me2Se)] .238
Fig. 12.2. Possible structural motifs of dirhodium tetracarboxylate compounds.
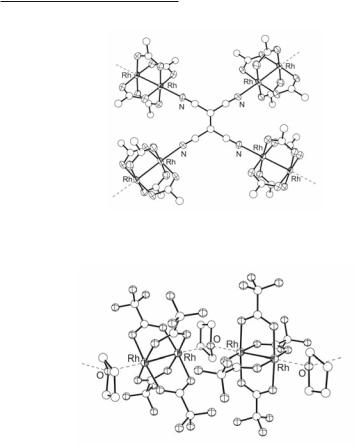
488Multiple Bonds Between Metal Atoms Chapter 12
Fig. 12.3. Molecular structure of [Rh2(O2CCF3)4]2(µ4-TCNE).
Fig. 12.4. A fragment showing the zig-zag chain structure [Rh2(O2CCF3)4(THF)] .
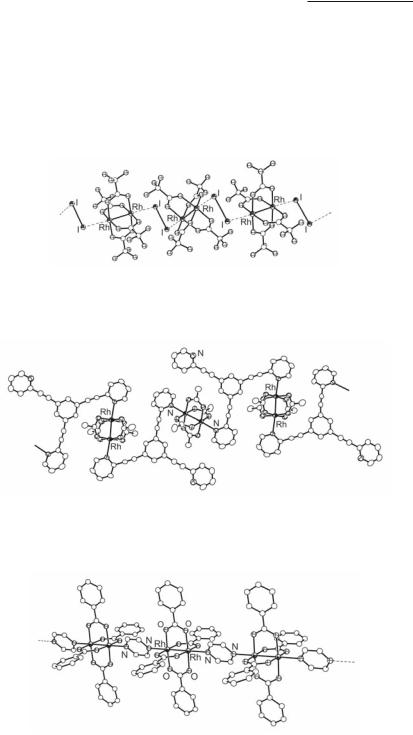
In the case of bidentate ligands L-L', the Rh2(O2CR)4 units form infinite linear or zigzag chains a (Fig 12.2), depending on the ligand and the hybridization of the donor atom. The ligand L-L' may employ the same donor atoms (L = L') such as in the cases of L-L' = PHZ (linear chain),155 DDA,155 NCPhCN,198 I2 (Fig. 12.5),54 S8,56 TCNE,202 DCNNQI and DM-DCNQI,203 4,4'-bpy,135 1,2-dimethoxy-4,5-bis[(2-pyridyl)ethynyl]benzene (dmpyethybz),150 1,3,5-tris[(2-pyridyl)ethenyl]benzene (tpyethebz; Fig. 12.6; zig-zag chain),305 2,6-bis- (N'-1,2,4-triazolyl)pyridine (btp),149 p-quinones,242 nickel biphenylbiguanide (linear chain),148 Cp*Ir(CN)3−,306 Fe(CN)6−,307 pyrazine (Fig. 12.7; linear chain),207,208 dimeric azine molecules,308 pyrazinecarboxylate compounds,309 [Rh2(µ-O2CCF3)2(CO)4] units (Fig. 12.8; linear chain),50,310 substituted ferrocene161,162 and ferrocenylphosphines.311 In addition, there are examples where the ax ligands have the same type of donor atoms but these are part of different chemical functionalities as in L-L' = AAMP (N-coordination through pyrimidine and aminomethyl N atoms),165 NITMe (O-coordination through nitrosyl and nitroxide oxygen atoms),219 ammpy (pyridine and amine N-coordination),147 and 3`-acetoxylanostan-11`-ol (hydroxy and acetoxy group O-coordination).241 The same donor atom may also coordinate to the metal at opposite
Rhodium Compounds 489
Chifotides and Dunbar
sides of the molecule (exo and endo sides), as in the corannulene58 (Fig. 12.9) and hemibuckminsterfullerene59 adducts. Ligand donor atoms of different identity L-L' (L & L') can be employed to link dimetal units, e.g., the N,O coordinated nitroxide radical IMMe,219 S,O-bound DMSO,51 p-quinones coordinated through the carbonyl group and the C=C double bond (Fig. 12.10)275,276 and PhNMe2 in the polymeric structure {Rh2{O2CC(CH3)2OC6H2(But)2OC(CH3)2CO2}2- (PhNMe2)} ;112 ax interactions in the dirhodium units of the latter are mediated through the nitrogen and p-carbon atoms of N,N'-dimethylaniline.112
Fig. 12.5. A fragment showing the alternating arrangement of Rh2(O2CCF3)4 and weakly coordinated diiodine molecules in {[Rh2(O2CCF3)4I2]·I2} .
Fig. 12.6. A fragment of the 1-D zig-zag chain [Rh2(O2CCH3)4(µ2-δ1:δ1- tpyethebz)·CH2Cl2] .
Fig. 12.7. A fragment of the linear chain structure [Rh2(O2CC6H5)4(pyz)2] .
490Multiple Bonds Between Metal Atoms Chapter 12
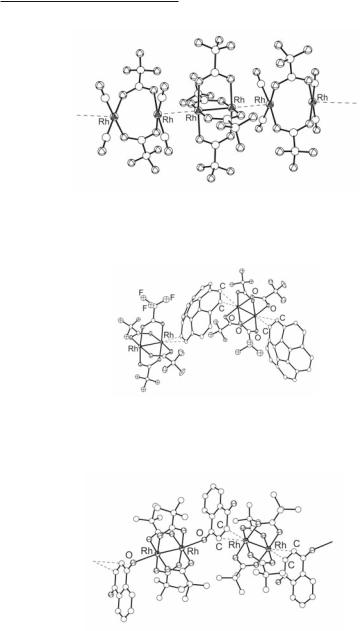
Fig. 12.8. A fragment of the arrangement of Rh2(O2CCF3)4 and Rh2(µ-O2CCF3)2(CO)4 units in the linear chain structure {Rh2(O2CCF3)4[Rh2(µ-O2CCF3)2(CO)4]2} .
Fig. 12.9. A fragment of the 1-D infinite chain structure {[Rh2(µ-O2CCF3)4]- (µ2-δ2:δ2-C20H10)} .
Fig. 12.10. A fragment of the infinite chain structure [Rh2(O2CCMe3)4(1,4-nq)] .
In the absence of exogenous donor ligands, dirhodium units generally are arranged so that the ax sites of each dimer associate with the oxygen atoms of an adjacent carboxylate group to form structures with infinite chains, such as those in [Rh2(O2CCH3)4] (Fig. 12.2b),312 [Rh2(O2CCF3)4] (Fig. 12.2b)47 or [Rh2(O2CC3H7)4] (Fig. 12.2c).298 These types of interactions are most likely present in other tetracarboxylate compounds, e.g., the linear chain alkanoates Rh2(O2CCnH2n+1)4 (n = 5, 7, or 11)63,64 and the alkoxybenzoates Rh2(O2CC6H4-4-OCnH2n+1)4 (n = 8-14).70 The latter compounds exhibit a conversion to a thermotropic discotic mesophase (i.e., a liquid crystalline phase) at 100-110 °C accompanied by structural changes from a crystalline compound to a
Rhodium Compounds 491
Chifotides and Dunbar
2-D rectangular or hexagonal columnar liquid-crystal phase.63,64,70,313,314 Additionally, there are several adducts wherein the Rh2(O2CR)4 core is associated with an adjacent Rh24+ unit through the O atoms of the carboxylate groups at one ax site only, whereas the opposite ax site is occupied by a different ligand L; such examples include the infinite chain {[Rh2(O2CCF3)4]3(µ2-DMSO-
S,O)2} (Fig. 12.11),51 the adduct (But4N)2[Rh8(O2CBut)16(µ2-δ1:δ1-O2CCH3)2]239 and the ‘dimers of dimers’ [Rh2(O2CCF3)4(THF)]2,49 [Rh2(O2CCF3)4(OCMe2)]2,315 [Rh2(O2CCH3)4(CNPh)]2,279
[Rh2(TiPB)n(O2CR)4-nL]2 (R = CH3, CF3, L = TiPBH, Me2CO, δ2-C6H5CH3, n = 2, 3),75 [Rh2(O2CCH3)4(PPh3)]2279 and {Rh2(O2CCH3)4[Ph2P(o-MeOC6H4)]}2.280 Despite the strong
affinity of Rh24+ tetracarboxylate complexes for ax ligands (illustrated by the aforementioned structural motifs), the synthesis of the paddlewheel dirhodium compound Rh2(TiPB)4 (Fig. 12.12), with no ax ligands, has been accomplished.16,75 The isolation of this complex as discrete, undimerized units is attributed to the presence of four sterically bulky 1,3,5-triisopro- pylphenyl groups which render impossible the association of each dirhodium unit with the carboxylate oxygen atoms of a neighboring Rh24+ core.16,75
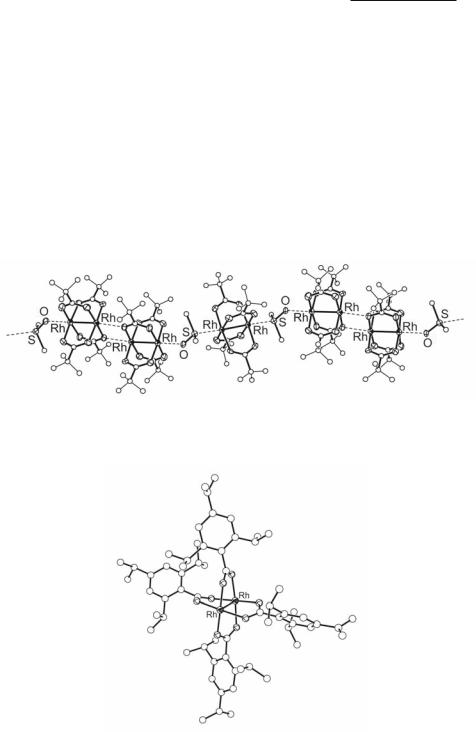
Fig. 12.11. A fragment showing the arrangement of Rh2(O2CCH3)4 and DMSO units in the extended structure {[Rh2(O2CCF3)4]3(µ2-DMSO-S,O)2} .
Fig. 12.12. Molecular structure of Rh2(TiPB)4.

492Multiple Bonds Between Metal Atoms Chapter 12
The ax Lewis bases of Rh2(O2CR)4 units may form 2-D sheets as in [Rh2(O2CCF3)4]2-
(µ4-TCNE),200 {[Rh2(O2CCF3)4]2(µ4-TCNQ)} ,201 [Rh2(O2CCH3)4]2[K3Co(CN)6],304 Na[Rh2- (µ-O2CH)4(µ3-δ1:δ1:δ1-O2CH)(H2O)]·H2O,235 and [Rh2(O2CCF3)4]3(µ3-δ2:δ2:δ2-C20H10)2,58 2-D extended organometallic networks as in {[Rh2(O2CCF3)4]3(µ4-δ2:δ2:δ2:δ1-C30H12)},59 pseudo
2-D architectures consisting of ribbon-type extended structures as those of [Rh2(O2CCF3)4(S8)]
(Fig. 12.13)56 and {[Rh2(O2CCF3)4]3(µ3-δ2:δ2:δ2-C18H12)2},57 pyramids as in {[Rh2(O2CCF3)4]3- CH3Si(C5H4N)3(δ1-C6H6)3},303 as well as other unusual supramolecular assemblies.149,303 More-
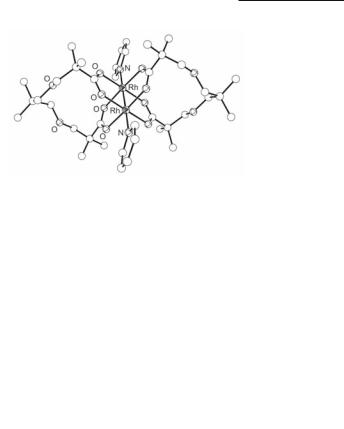
over, diacids have been employed as building blocks to prepare macrocyclic dimers with transoid arrangement of the bridging ligands,113 covalently linked ‘dimers of dimers’ that form square motifs,115 and layered hexagonal networks of dirhodium units with chelating carboxylate groups.112 The assembling of chelating110 (Fig. 12.14) or linear diacids has produced a variety of molecular boxes with dirhodium units at the corners of the macrocycles;111,114 the latter are discussed in Section 12.7.2. A notable reversible phase transition that generates 1-D channels takes place upon cooling samples of the host molecule [Rh2(O2CC6H5)4(pyz)2] in a CO2 atmosphere.208
The impact of a pure electronic change in the character of the metal atom on the preference of the ligand donor atoms is nicely demonstrated by the Rh2(O2CR)4 adducts with the ambidentate ligand DMSO. For Rh2(O2CR)4 adducts with DMSO, when R = CH3, C2H5 and C6H5, the electron-donating substituents result in coordination to the sulfur atom,226,227,248 whereas for R = CF3, bonding changes to the oxygen atom.227 The gas phase reaction between DMSO and Rh2(O2CCF3)4, however, affords {[Rh2(O2CCF3)4]3(µ2-DMSO-S,O)2} (Fig. 12.11) and [Rh2(O2CCF3)4]7(DMSO)8 with S- and O-bound DMSO. Another example that supports the control of ligand coordination to Rh2(O2CR)4, by changing the effective electronegativity of the carboxylate R group substituent, is that of 1,4-benzoquinone: for R = CF3 ligation occurs only through the O atoms of the p-quinone carbonyl groups,242 whereas for R = But, the increase in the ‘softness’ of the Rh metal atoms enables coordination to the C=C double bond of p-quinone.275 Other important interactions that determine the preferential ligand binding sites include intramolecular hydrogen-bonds between the substituents of the ligand and the O atoms of the bridging carboxylate; such interactions have been encountered in adducts with various pyridine, pyrimidine and triazine derivatives,145,146,148 1-methyladenosine,184 and azathioprine.188 This binding site preference is clearly demonstrated in Rh2(O2CCH3)4(damt)2 (12.12); although damt has four nitrogen atoms that are potential binding sites, ax binding occurs through the site that favors formation of intramolecular hydrogen bonds between the exocyclic amino groups and the acetate O atoms.146 The impact of these interactions is further demonstrated by biologically relevant compounds discussed in Section 12.7.3.
Fig. 12.13. The pseudo 2-D ribbon-type structure of [Rh2(O2CCF3)4(S8)] .
Rhodium Compounds 493
Chifotides and Dunbar
Fig. 12.14. Molecular structure of Rh2{[O2CC(CH3)2CH2OCH2]2C(CH3)2}(py)2.
In summary, characterization of dirhodium carboxylate compounds by X-ray crystallography has provided important information about the molecular structures of these compounds. These data are essential for interpretation of their spectroscopic properties and electronic struc- tures19,316-334 which are discussed in Chapter 16.
12.12
12.3 Other Dirhodium Compounds Containing Bridging Ligands
12.3.1 Complexes with fewer than four carboxylate bridging groups
Mixed carboxylate complexes of the type Rh2(O2CCH3)4-n(O2CR)n, n = 2 or 3, include those for which R = CPh3,38 C6H2-2,4,6-(p-tol)3,39 C6H4-2-OH,72,335 and 1,3,5-triisopropylphenyl,75 as well as Rh2(O2CCF3)4-n(O2CR)n, n = 2 or 3 for R = TiPB.75 During exchange reactions of Rh2(O2CR)4 carboxylate ligands, a stepwise replacement of RCO2- by R'CO2- occurs with retention of the Rh24+ core. Monitoring the displacement of CH3CO2- with CF3CO2- by NMR spectroscopy has established that the rate constants for the first, second, third and fourth substitution reactions are in the approximate ratio 1:2:0.1:0.025,36 an indication that there is a marked preference for the cis-Rh2(O2CCH3)2(O2CCF3)2 isomer. This is in accordance with the prevalence and stability of compounds that contain the bis-carboxylate unit cis-[Rh2(O2CR)2]2+ (Table 12.2). The lability of carboxylate ligands is further demonstrated by the large number of neutral and cationic Rh24+ compounds with fewer than four carboxylate groups. Structural data for these compounds are provided in Table 12.2.
Table 12.2. Structural data for tris, bis and mono carboxylato Rh24+ compounds
|
|
|
|
|
|
|
|
|
Compound |
|
r (Rh–Rh)a (Å) |
r (Rh–Lax) (Å)b |
Donor |
ref. |
|
|
|
|
|
|
|
|
|
|
atom(s) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tris-carboxylato compounds |
|
|
|
|
[Rh2(µ-O2CCH3)3(py)4]CF3SO3 |
|
|
2.473(1) |
2.25(1) |
N |
367 |
||||||||
[Rh2(µ-O2CCH3)3(py)4]CF3SO3 |
|
|
2.474(1) |
2.253(3) |
N |
368 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.235(4) |
|
|
Rh2(µ-O2CCH3)3(δ2-O2CCH3)(bpy)c |
|
2.475(1) |
2.12(1)d |
N |
372,373 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.466(8)e |
O |
|
[Rh2(µ-O2CCH3)3(δ4-bpnp)]PF6 |
|
|
2.405(2) |
2.20(1) |
N |
382,383 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.19(1) |
|
|
|
|
|
|
|
|
|||||||||
{[Rh2(µ-O2CCH3)3]2(µ2-δ4:δ4-L1)}(PF6)2f |
|
2.40g |
2.16g |
N |
384 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.31g,h |
|
|
Rh2(µ-O2CCH3)3(O-TMPP)(MeOH)·EtOH |
|
2.423(1) |
2.351(2)i |
O |
565,566 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.251(2)i |
|
|
[Rh |
2 |
(µ-O |
2 |
CCH |
3 |
) |
3 |
(O-MPP)](HO |
CCH ) |
|
2.421(1) |
2.300(5)j |
O |
516 |
|
|
|
|
2 |
3 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
2.342(4)j |
|
|
Rh2(µ-O2CCH3)3(O-MPP)(NCCH3) |
|
2.421(1) |
2.373(3)k |
O |
568 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.203(4) |
N |
|
[Rh2(µ-O2CCH3)3{PhP(C6H4)(o-BrC6H4)}P(C6H11)3]·CHCl3 |
|
2.477(1) |
2.400(1) |
P |
530 |
|||||||||
Rh2(µ-O2CCH3)3[PhP(C6H4)(o-ClC6H4)](HO2CCH3)2 |
|
2.410(1) |
2.378(6) |
O |
531 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.26(1) |
|
|
Rh2(µ-O2CCH3)3{PhP(C6H4)(o-BrC6H4)}(HOCCH3)2 |
|
2.432(1) |
2.273(4) |
O |
532 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.434(4) |
|
|
|
|
|
|
|
|
|||||||||
Rh2(µ-O2CCH3)3{(p-CH3OC6H3)P(p-CH3OC6H4)2}(HO2CCH3)2 |
|
2.421(1) |
2.341(4) |
O |
533 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.257(4) |
|
|
|
|
|
|
|
|
|||||||||
{Rh2(µ-O2CCH3)3[Ph2P(C6H4)]}(HO2CCH3)2 |
|
2.430(2) |
2.336(4) |
O |
524,525 |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
2.301(4) |
|
|
|
494 |
|
12 Chapter |
|
Bonds Multiple |
|
||
|
|
Atoms Metal Between |
