
Multiple Bonds Between Metal Atoms / 12-Rhodium Compounds
.pdf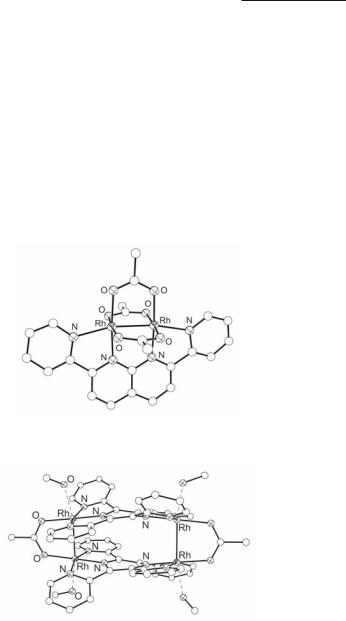
Rhodium Compounds 505
Chifotides and Dunbar
exhibit rich electrochemistry: in addition to electrochemical processes corresponding to oxidation to the Rh25+ core, they exhibit two reduction processes,382,386 which have been studied by EPR spectroscopy389 and single-point energy theoretical calculations.388 The carboxylate groups of the tetradentate 1,8-naphthyridine-2,7-dicarboxylate ligand (dcnp) presumably occupy ax positions in Na[Rh2(µ-O2CCH3)3(dcnp)].390 The bpa ligands (12.16), in the two isomers of [Rh2(µ-O2CCH3)2(δ3-bpa)2]2+ (C2 and Cs symmetry point groups), are coordinated in a tridentate fashion to each Rh atom.206 It is notable that, among the disubstituted complexes, the carboxylate groups are usually found in the cisoid arrangement, except for trans-(2,2)Rh2(µ- O2CCH3)2(mhp)2(Im)391 (mhp: anion of 6-methyl-2-hydroxypyridine); the latter is discussed with the monocarboxylate adduct trans-[Rh2(µ-O2CCH3)(chp)2(NCCH3)3]BF4392 (chp: anion of 6-chloro-2-hydroxypyridine) in section 12.3.2. Likewise, mixed carboxylate/formamidinate and carboxylate/9-ethylguanine complexes393-395 are discussed in Sections 12.3.3 and 12.7.3, respectively.
Fig. 12.18. The cation in [Rh2(µ-O2CCH3)3(δ4-bpnp)]PF6.
Fig. 12.19. The structure of the cationic rectangle [Rh4(µ-O2CCH3)2(µ2-δ3:δ3-tppz)2(MeOH)4]4+.
12.3.2Complexes supported by hydroxypyridinato, carboxamidato and other (N, O) donor monoanionic bridging groups
The quest for bridging ligands that preclude ax interactions led to the introduction of 6-X-oxopyridinate bridging groups (X = Me, F, and Cl; 12.4).391 In general, the Rh–Rh bond lengths for tetrahydroxypyridinato compounds vary between 2.36 Å and 2.41 Å, which is within the range of Rh–Rh distances for most tetracarboxylate complexes. The Rh–Rh bond distance in Rh2(mhp)4 (2.359(1) Å)396,397 is comparable to that of the tetracarboxylate complex Rh2(TiPB)4 (Rh–Rh = 2.350(1) Å)16 and to that of the pyrazolate bridged compound

506Multiple Bonds Between Metal Atoms Chapter 12
Rh2(3,5-Me2pz)4(NCCH3)2 (Rh–Rh = 2.353(3) Å),398 which are among the shortest recorded Rh–Rh bond distances. The longest Rh–Rh bond distance among the compounds of this class is exhibited by Rh2(fhp)4(DMSO) (X = F; Rh–Rh = 2.410(1) Å).399 The significant increase in the Rh–Rh bond distance, as compared to Rh2(chp)4 (X = Cl; 2.379(1) Å),391 is attributed mainly to the presence of the S-bound molecule of DMSO in the ax position, as well as to the electron withdrawing effect of the fluorine atoms.
The 6-X-oxopyridinate derivatives are prepared by one of the following procedures: reaction of the sodium salt, e.g., Na(mhp)396 or Na(fhp),399 with RhCl3·xH2O; reaction of Na(mhp) with Rh2(O2CCH3)4(MeOH)2,396 or reaction of the molten ligands Hhp, Hmhp or Hchp with
Rh2(O2CCH3)4.391,400
Homoleptic Rh24+ paddlewheel compounds supported by (N, O) 6-X-oxopyridinate bridging groups (X = Me, F, and Cl) (12.4) as well as other (N, O) donor groups (12.5-12.6) exhibit structural diversity. The crystal structures of a number of these compounds (Table 12.3) indicate that they exhibit four possible geometric isomers designated as cis-(2,2), trans-(2,2), (3,1) and (4,0) (Fig. 12.20).
Fig. 12.20. Possible orientations of asymmetric bridging groups around the Rh2 core and symmetry of the immediate coordination sphere.
Table 12.3. Structural data for Rh24+ compounds supported by carboxamidato and other (N, O) donor bridging groups
Compound |
r (Rh–Rh)a |
r (Rh–Lax)b |
Donor |
ref. |
|
(Å) |
(Å) |
atom(s) |
|||
|
|
||||
trans-(2,2)-Rh2(mhp)4 |
2.359(1) |
c |
c |
396,397 |
|
|
|
||||
trans-(2,2)-Rh2(mhp)4·H2O |
2.370(1) |
c |
c |
391 |
|
|
|
||||
trans-(2,2)-[Rh2(mhp)4]·CH2Cl2 |
2.367(1) |
c |
c |
285,401 |
|
|
|
||||
(3,1)-Rh2(mhp)4(NCCH3) |
2.372(1) |
2.152(7) |
N |
391 |
|
(3,1)-[Rh2(mhp)4(Im)]·0.5CH3CN |
2.384(1) |
2.144(4) |
N |
391 |
|
(3,1)-[Rh2(mhp)4]2·2CH2Cl2d |
2.369(1) |
2.236(3)e |
O |
402 |
|
(3,1)-[Rh2(mhp)4(Hmhp)]·0.5C6H5CH3 |
2.383(1) |
2.195(4) |
O |
402 |
|
[Rh2(mhp)3(µ-OTs)]2·Et2Od |
2.377(3) |
2.24(1)e |
O |
405 |
|
|
2.376(3) |
2.30(2)e |
|
|
|
trans-(2,2)-Rh2(chp)4 |
2.379(1) |
c |
c |
391 |
|
|
|
||||
(3,1)-[Rh2(chp)4(Im)]·3H2O |
2.385(1) |
2.129(9) |
N |
391 |
|
trans-[Rh2(µ-O2CCH3)(chp)2(NCCH3)3]BF4 |
2.444(1) |
2.149(6) |
N |
392 |
|
(4,0)-Rh2(fhp)4(DMSO) |
2.410(1) |
2.332(3) |
S |
399 |
|
Rh2(fhp)4(THF)f |
2.34g |
f |
O |
399 |
|
(3,1)-[Rh2(hq)4(py)]·1.5C2H4Cl2 |
2.396(1) |
2.140(3) |
N |
404 |
|
trans-Rh2(µ-O2CCH3)2(mhp)2(Im) |
2.388(2) |
2.17(1) |
N |
391 |
|
trans-[Rh2(µ-O2CCH3)2(mhp)2(Im)]·2CH2Cl2 |
2.388(1) |
2.133(7) |
N |
391 |

Rhodium Compounds 507
Chifotides and Dunbar
Compound |
r (Rh–Rh)a |
r (Rh–Lax)b |
Donor |
ref. |
|
(Å) |
(Å) |
atom(s) |
|||
|
|
||||
cis-[Rh4(mhp)4(µ2-Cl)4(PhCN)2] |
2.537(3) |
2.13(2) |
N |
197 |
|
|
|
|
|
|
|
cis-(2,2)-[Rh2(pyro)4(Hpyro)2]·2CH2Cl2 |
2.445(1) |
2.325(1) |
O |
430 |
|
cis-(2,2)-[Rh2(vall)4(Hvall)2]·2Hvall |
2.392(1) |
2.357(3) |
O |
430 |
|
[Rh2(mphonp)4]·C6H5OCH3·½Et2O |
2.566(3) |
2.46(2) |
Nh |
406 |
|
|
|
2.42(2) |
|
|
|
cis-(2,2)-[Rh2(HNCOCH3)4(H2O)2]·3H2O |
2.415(1) |
2.352(2) |
O |
414 |
|
cis-(2,2)-[Rh2(HNCOCH3)4(DMSO)2]·H2O |
2.452(1) |
2.414(1) |
S |
422 |
|
{[Rh2(HNCOCH3)4]3(µ3-Cl)2·4H2O} i |
2.422(1) |
2.552(1) |
Cl |
412 |
|
|
2.424(1) |
2.554(1) |
|
|
|
|
2.431(1) |
2.610(1) |
|
|
|
{[Rh2(HNCOCH3)4](µ4-I)·6H2O} j |
2.438(1) |
2.975(1) |
I |
429 |
|
[Rh2(µ-HNOCCH3)3(µ-O2CCH3)(DMSO)2]·2H2O |
2.446(1) |
2.413(1) |
S |
421 |
|
Rh2(µ-HNCOCH3)3(µ-O2CCH3)(AsPh3)2 |
2.467(3) |
2.553(4) |
As |
423 |
|
Rh2(µ-HNCOCH3)3(µ-O2CCH3)(SbPh3)2 |
2.461(2) |
2.699(3) |
Sb |
423 |
|
trans-(2,2)-Rh2(PhNCOCH3)4(NCPh)2 |
2.422k |
2.205k |
N |
420 |
|
|
|
2.248k |
|
|
|
(3,0)-Rh2(PhNCOCH3)4(DMSO) |
2.397(1) |
2.395(1) |
S |
409 |
|
cis-(2,2)-Rh2(PhNCOCH3)4(DMSO)2 |
2.448(1) |
2.606(2) |
S |
409 |
|
|
|
2.566(2) |
|
|
|
cis-(2,2)-Rh2(HNCOCH3)4(AsPh3)2 |
2.471(2) |
2.540(2) |
As |
423 |
|
cis-(2,2)-Rh2(HNCOPh)4(py)2 |
2.437(1) |
2.227(7) |
N |
425 |
|
cis-(2,2)-[Rh2(HNCOPh)4(SbPh3)2]·CH2Cl2 |
2.463(1) |
2.681(1) |
Sb |
425 |
|
cis-(2,2)-Rh2(HNCOCF3)4(py)2 |
2.472(3) |
2.26(1) |
N |
417 |
|
|
|
2.31(1) |
|
|
|
{Rh2(HNCOCF3)4(4,4'-bpy)} |
2.456(1) |
2.222(4) |
N |
426 |
|
trans-[Rh2(µ-O2CCH3)2(µ-HNCOCF3)2- |
2.430k |
2.274(8) |
N |
411 |
|
(9-MeAdeH2)2](NO3)2l |
|
|
|
|
|
trans-[Rh2(µ-O2CCH3)2(µ-HNCOCF3)2- |
2.427k |
2.283(3) |
N |
411 |
|
(9-EtGuaH)2]·2MeOH·2H2O |
|
|
|
|
|
Rh2(HNCOCF3)4(Guo)2·3H2O |
2.459k |
2.27(1) |
N |
411 |
|
|
|
2.30(1) |
|
|
|
trans-[Rh2(µ-HNCOCH3)2(µ-HNCOCF3)2- |
2.432k |
2.296(7) |
N |
411 |
|
(Guo)2]·3H2O |
|
2.280(7) |
|
|
|
[Rh2(HNCOCF3)4(dGuo)2]·3H2O |
2.475k |
2.326k |
N |
411 |
|
|
|
2.250k |
|
|
|
[Rh2(HNCOCF3)4(Ino)2]·3H2O |
2.455k |
2.35(1) |
N |
411 |
|
|
|
2.27(1) |
|
|
|
[Rh2(HNCOCF3)4(cyd)] m |
2.463(1) |
2.326(5)n |
N |
410 |
|
|
|
2.247(5)o |
O |
|
|
[Rh2(HNCOCF3)4(1-Mecyd)2]·2H2O |
2.469(1) |
2.354(2) |
N |
410 |
|
cis-[Rh2(µ-HNCOCF3)2(phen)2(py)2](PF6)2·Et2O |
2.612(1) |
2.238(5) |
N |
356 |
|
|
|
2.241(5) |
|
|
|
cis-Rh2(µ-HNCOCF3)2(phen)2Cl2 |
2.614(1) |
2.540(2) |
Cl |
427 |
|
Homochiral Carboxamidate Compounds |
|
|
|||
cis-(2,2)-Rh2(5R-MEPY)4(NCCH3)2(PriOH) |
2.457(1) |
2.215(4) |
N |
431 |
|
|
|
2.236(4) |
|
|
|

508 Multiple Bonds Between Metal Atoms
Chapter 12
Compound |
r (Rh–Rh)a |
r (Rh–Lax)b |
Donor |
ref. |
|
(Å) |
(Å) |
atom(s) |
|||
|
|
||||
Rh2(5S-dFMEPY)4(CH3CO2CH2CH3)2, |
2.467(1) |
2.362k,q |
O |
432 |
|
Rh2(5S-dFMEPY)4(CH3CO2CH2CH3)(H2O)·1.5H2Op |
|
2.324k,q |
|
|
|
|
|
2.286k,q |
|
|
|
|
|
2.325k,r |
|
|
|
cis-(2,2)-Rh2(5S-DMAP)4(NCCH3)2·CH3CN·6H2O |
2.454(1) |
2.231(4) |
N |
433 |
|
|
|
2.224(4) |
|
|
|
cis-(2,2)-Rh2(4S-BNAZ)4(NCCH3)2 |
2.533(1) |
2.210k |
N |
434 |
|
cis-(2,2)-Rh2(5S-BNOX)4(NCCH3)2·CH3CN |
2.472(2) |
2.205(8) |
N |
431 |
|
|
|
2.252(8) |
|
|
|
cis-(2,2)-Rh2(4S-PHOX)4(NCCH3)2·2CH3CN |
2.471(1) |
2.19(1) |
N |
435 |
|
cis-(2,2)-[Rh2(4S-MEOX)4(NCPh)2](C6H5CN)2 |
2.477(1) |
2.191(2) |
N |
436 |
|
cis-(2,2)-Rh2(4S-THREOX)4(NCPh)2 |
2.474(1) |
2.203(2) |
N |
436 |
|
cis-(2,2)-Rh2(4S-MACIM)4(NCCH3)2·2CH3CN |
2.459(1) |
2.220(3) |
N |
437 |
|
(3,1)-Rh2(4S-MACIM)4(NCCH3)2·2CH3CN |
2.460(1) |
2.223(4) |
N |
437 |
|
(4,0)-Rh2(4S-MACIM)4(NCCH3)2 |
2.445(1) |
2.179(4) |
N |
438 |
|
|
|
2.268(4) |
|
|
|
cis-(2,2)-Rh2(4S-MBOIM)4(NCCH3)2·2CH3CN |
2.461(1) |
2.210(3) |
N |
437 |
|
cis-(2,2)-Rh2(4S-MPPIM)4(NCCH3)2·2CH3CN |
2.464(1) |
2.219(4) |
N |
437 |
|
cis-(2,2)-Rh2(4S-MCHIM)4(NCCH3)2·2CH3CN |
2.451(1) |
2.216(3) |
N |
437 |
|
cis-(2,2)-Rh2(S,S-MANIM)4(NCCH3)2 |
2.467k |
2.206k |
N |
439 |
|
cis-(2,2)-Rh2(S,R-NaphthAZ)4(NCCH3)2 |
2.529(3) |
2.22[2] |
N |
440 |
a Distances are given with up to 3 decimal digits.
bIn some cases the average Rh–L bond lengths are quoted. In these instances the estimated deviation, which is given in square brackets, is calculated as [ ] = [Ψn¨i2/n(n < 1)]1/2, in which ¨i is the deviation of the ith of n
|
values from the arithmetic mean of the set. |
c |
No ax ligands. |
d |
‘Dimer of dimers’. |
e |
Rh–O distance to mhp O atom of neighboring dirhodium unit. |
f |
Unsatisfactory solution of crystal structure; only unit cell determined. |
g |
Average approximate distance. |
h |
Nitrogen atoms of mphonp ligand. |
i |
Honeycomb arrangement of Rh24+ and Rh25+ units bridged by µ3-Cl< ions. |
j |
Diamondoid arrangement of Rh24+ and Rh25+ units bridged by µ4-I< ions. |
k |
Esds not reported. |
l |
Each 9-methyladenine molecule is protonated at position N1 of the purine ring. |
m One dimensional zig-zag chain. |
|
n |
Distance to the ring nitrogen N(3) of cytosine. |
o |
Distance to the keto O(2) site of cytosine. |
p |
The two molecules co-crystallize in the same crystal with 1.5 interstitial H2O molecules. |
q |
Rh–O distance to ax molecule of CH3CO2CH2CH3. |
r |
Rh–O distance to ax molecule of H2O. |
The compounds Rh2(mhp)4,396,397 Rh2(mhp)4·H2O,391 [Rh2(mhp)4]·CH2Cl2285,401 and with D2d molecular symmetry (i.e., symmetrical trans-(2,2) arrangement;
Fig. 12.20b) lack ax ligands due to the presence of two 6-X hp bridging substituents located near each ax site; in the case of the hydrate, the H2O molecules engage in hydrogen bonding with the mhp molecules. The (3,l) arrangement (Fig 12.20c) is encountered in the adducts Rh2(mhp)4L, (L = CH3CN391 or imidazole (Im),391 Hmhp402), Rh2(chp)4(Im),391 as well as in the ‘dimer of dimers’ [Rh2(mhp)4]2.402 The (3,1) arrangement permits binding of an ax ligand to the rhodium atom with the fewest N atoms coordinated to it (12.19), but in this case, the

Rhodium Compounds 509
Chifotides and Dunbar
other ax site is even more blocked as compared to the (2,2) arrangement, which precludes ax ligands from occupying this position. The [Rh2(mhp)4]2 structure402 provides an example of the (3,1) arrangement wherein the molecules, which are denied access to other coordinating ligands, associate with the O atom from an mhp bridging group of an adjacent dirhodium unit. This association is evidenced by the 103Rh NMR spectra of trans-(2,2)-Rh2(mhp)4 and (3,1)- [Rh2(mhp)4]2; the former exhibits a singlet at β = +5745 ppm, whereas the dimer exhibits a pair of doublets centered at β ~ +7644 ppm and ~ +4322, due to the two nonequivalent 103Rh nuclei with 1J(103Rh, 103Rh) coupling of ~35 Hz.403 Another example of the (3,1) arrangement is that of the 2-quinolinol (Hhq) adduct Rh2(hq)4(py), which has an ax py ligand coordinated to the Rh atom with the least steric hindrance.404 The option of a single ax ligand is apparent in trans-Rh2(µ-O2CCH3)2(mhp)2(Im) (Fig. 12.21), wherein not only are the acetate groups found in the unusual transoid arrangement, but the two mhp ligands point in the same direction, thus preventing ax coordination to one rhodium atom while leaving the other ax site accessible to the imidazole ligand.391 In the ‘dimer of dimers’ [Rh2(mhp)3(µ-OTs)]2, which is obtained from the reaction of Rh2(mhp)4 with toluene-p-sulfonic acid (TsOH), the ‘open’ ax sites are involved in intermolecular Rh···O(mhp) interactions (2.24(1) Å and 2.30(2) Å)405 similar to those encountered in the ‘dimer of dimers’ (3,1)-[Rh2(mhp)4]2.402
12.19 |
12.20 |
Fig. 12.21. Molecular structure of trans-Rh2(O2CCH3)2(mhp)2(Im).
The polar (4,0) ligand arrangement 12.20 is found in the fhp complexes Rh2(fhp)4L, L = EtOH, THF or DMSO,399 which structurally resemble the Cr, Mo, and W analogs. In a similar vein to the (3,l) arrangement 12.19, the ax ligands L bind to the rhodium atom with the fewest N atoms coordinated to it (in this case none), and the additional ax bond stabilizes the structure. It appears that steric effects are important in determining the type of isomer preferred. For example, it is less difficult to place four small fluorine atoms in the (4,0) arrange-

510Multiple Bonds Between Metal Atoms Chapter 12
ment without creating significant repulsion, whereas four large chlorine or methyl groups would result in unfavorable repulsive interactions.399 There is no general method, however, for predicting which isomer will be preferred, and the outcome depends on the interplay of various weak non-bonding attractions and repulsions. This argument is further supported by the polar arrangement of the chp ligands in trans-[Rh2(µ-O2CCH3)(chp)2(NCCH3)3]BF4, despite the fact that the two chlorine atoms of the chp pairs make contacts close to the sum of the van der Waals radii.392
In the cage-like structure cis-[Rh4(mhp)4(µ2-Cl)4(PhCN)2], the two dirhodium units are linked by bridging chloride ions, the mhp ligands are in the usual cis-(2,2) arrangement, and the ax ligands are bound to the less hindered sites.197 Unexpected binding modes are observed in the complex Rh2(mphonp)4 (Hmphonp = 5-methyl-7-phenyl-1,8-naphthyridin-2-one; 12.21), which contains two bridging and two chelating mphonp anions in the unusual (N, C) mode (involving cyclometalation of the naphthyridine rings) as depicted in 12.22.406
12.21 |
12.22 |
Interest in the compounds Rh2(O2CR)n(R'NCOR)4-n (R = CH3 or CF3; R' = H or Ph; n = 0-3) supported by another class of mixed (N, O) donor anionic ligands, namely the carboxamidates (12.5), stems from the rich electrochemistry exhibited by these complexes due to the higher electron density of the Rh(II) centers as n increases.407-409 The structural versatility of these compounds is notable, owing to the concomitant presence of hydrogen-bonding donor and acceptor sites on the bridging groups410-412 (carboxylate groups function only as acceptors). Dirhodium compounds with carboxamidate bridging groups that have been studied by X-ray crystallography are listed in Table 12.3.
Reactions of Rh2(O2CCH3)4 with molten acetamide, trifluoroacetamide and N-phenylacet- amide have been employed to prepare the fully substituted complexes Rh2(HNCOCH3)4,407,413-415 Rh2(HNCOCF3)4,416,417 and Rh2(PhNCOCH3)4,409,418 respectively. Partially substituted complexes are simultaneously formed and must be separated by liquid chromatography. Refluxing Rh2(O2CCH3)4 with acetamide in anhydrous chlorobenzene in a Soxhlet extraction apparatus (in the presence of sodium carbonate) affords only the tetra-substituted cis-(2,2)- Rh2(HNCOCH3)4.419 The compound Rh2(HNCOCF3)4 is prepared by a molten reaction; the cis-(2,2) isomer was identified by X-ray diffraction studies of its bis(pyridine) analog as the most abundant product (>94%), whereas the next most abundant fraction (4%) is the (3,1) isomer, based on 19F NMR spectroscopy.417 These results indicate that the cis-(2,2) isomer (Fig 12.20a) is the most stable, although differences in free energy among the other isomers are small. Both the cis-(2,2) and (3,1) isomers of Rh2(PhNCOCH3)4 have been synthesized (by molten reactions), separated by HPLC and subsequently crystallized as their DMSO adducts cis-(2,2)-Rh2(PhNCOCH3)4(DMSO)2 (Fig. 12.22) and (3,0)-Rh2(PhNCOCH3)4(DMSO); both adducts contain S-bound DMSO.409 It is notable that the first N-substituted trans-(2,2)- Rh2(PhNCOCH3)4(NCPh)2 isomer has been synthesized (by using the Soxhlet extraction method) and structurally characterized.420 The presence of two ax ligands in this adduct is reasonable due to the orientation of the phenyl rings attached to the N atoms of the bridging groups: the
Rhodium Compounds 511
Chifotides and Dunbar
rings are nearly perpendicular to the plane of the amidate bridging groups to avoid steric repulsion of their ortho protons with the CH3 groups of the acetamide moieties.
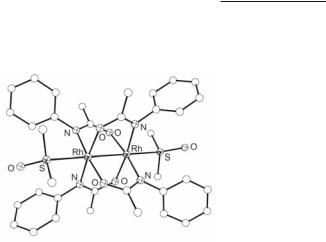
Fig. 12.22. Molecular structure of cis-(2,2)-Rh2(PhNCOCH3)4(DMSO)2.
Both acetamidate and trifluoroacetamidate complexes as well as the mixed carboxamidate/ acetate complexes readily form adducts with H2O,414 DMSO,421 pyridine,413,417 acetonitrile,408,422 PPh3,423 AsPh3,423 and SbPh3.423 The stability constants of the CO adducts with Rh2(O2CCH3)n(R'NCOCH3)4-n (n = 0, 2, 3, 4) increase, whereas the frequency of the ι(CO) stretching mode decreases with increasing n, due to an increase in the degree of /-backbonding; these observations corroborate the enhanced electron-donating ability of acetamidate compared to carboxylate groups.424 This is also supported by the formation constants of the ligand exchange reactions involving displacement of CH3CN by DMSO, which indicate that different modes of DMSO binding (S vs O) exist for [Rh2(O2CCH3)4-n(R'NCOCH3)n]0/+ as a function of the value n and the oxidation state.422 Structural studies of the series of compounds Rh2(O2CCH3)(HNCOCH3)3L2, where L = DMSO,421 AsPh3423 or SbPh3423 show them to be isostructural with each rhodium atom formally bound to a cis pair of carboxamide nitrogen atoms.
The benzamidate (12.5; R = Ph, R' = H) derivative Rh2(HNCOPh)4 readily forms adducts with benzamide, PPh3, pyridine and SbPh3; the crystal structure determinations of the compounds with the latter two ligands reveal that they possess the common cis-(2,2) geometry (Fig. 12.20a).425 Polymeric adducts of Rh2(HNCOCF3)4 with pyrazine, 1,4-diazabicyclo[2.2.2]octane and 4,4'-bipyridine have been prepared, and the compound {Rh2(HNCOCF3)4(4,4'-bpy)} has been structurally characterized.426 The bis-trifluoroacetamidate compounds cis-[Rh2- (µ-HNCOCF3)2(phen)2(py)2](PF6)2356 and cis-Rh2(µ-HNCOCF3)2(phen)2Cl2427 with two chelating N-N groups occupying eq positions, and Rh2(HNCOCF3)4 adducts with 2,4-diaminopyrimidine ligands428 have been prepared. The unusual compound {[Rh2(HNCOCH3)4]3(µ3-Cl)2} , which consists of Rh24+ and Rh25+ units bridged by chloride ions in a honeycomb arrangement, has low conductivity.412 Alternatively, the diamondoid network of {[Rh2(HNCOCH3)4]2(µ4-I)·6H2O} units undergoes reversible dehydration-rehydration cycles of the interstitial water molecules with a 105 enhancement of its electrical conductivity in the hydrated form, most likely due to deformation of the hydrogen-bond network and localization of the odd electrons on some of the Rh2 sites in the dehydrated form.429 The carboxamidate adducts with DNA nucleobases and their nucleosides410,411 are discussed in Section 12.7.3, and their electrochemical properties as well as their Rh25+ counterparts are presented in Section 12.6.
Several dirhodium complexes with (N, O) donor sets in which the nitrogen atom is incorporated into five membered rings are those with 2-pyrrolidinone (Hpyro) and β-valerolactam

512Multiple Bonds Between Metal Atoms Chapter 12
(2-piperidinone, Hvall) (12.6); their adducts Rh2(pyro)4(Hpyro)2 and Rh2(vall)4(Hvall)2 have been prepared from Rh2(O2CCH3)4 by ligand exchange of the acetate groups, and exhibit the usual cis-(2,2) arrangement (Fig 12.20a).430 In weakly coordinating solvents, CO binding to Rh2(pyro)4 and Rh2(vall)4 is fast and CO dissociation is very slow, but in solvents such as CH3CN, CO binding is reversible.430 Homochiral dirhodium carboxamidate compounds431-441 find extensive application in catalysis and are discussed in detail in Chapter 13.
12.3.3 Complexes supported by amidinato and other (N, N) donor bridging groups
Among the common monoanionic bridging ligands are N-donor bidentate amidinate groups (12.7), which have emerged as one of the more important classes. Amidinate bridging groups introduce chemical and structural diversity to dinuclear complexes, resulting in rich electrochemistry,442 improvement of their biological activity,443 and fine control in the design of supramolecular assemblies.13,15 The robust nature and the strong trans influence of the amidinate bridging groups render the behavior of this class of compounds different, in many aspects, from that of the carboxylate series.
The parent compound of the formamidinate series, Rh2(DPhF)4 (12.7; R = H, Ar = Ph), is prepared by reaction of Rh2(O2CCH3)4 with molten HDPhF at 130 ºC,444 a reaction that generally is applicable to the preparation of various formamidinate analogs.442 An alternative method of preparation involves refluxing RhCl3 with the neutral formamidine in EtOH/Et3N, but the yields are better with the former method, especially for ligands with lower melting points; compounds with ArNCHNAr− bridging groups, Ar = XC6H4 (X = p-OMe, p-Me, H, m-OMe, p-Cl, m-Cl, m-CF3, p-CF3) or Ar = 3,4-Cl2C6H3, 3,5-Cl2C6H3, have been synthesized by the previous methods.442 The reaction of Rh2(DTolF)2(O2CCF3)2(H2O)2445 with molten N,N'- di-p-tolylformamidine (HDTolF) at 135 °C affords Rh2(DTolF)4.446 The analogous compound Rh2(DPhBz)4 (Fig. 12.23) is obtained from the reaction of Rh2(O2CCH3)4 with benzamidine (12.7; R = Ph, Ar = Ph).447,448 Unlike Rh2(O2CCH3)4(CO)2, which is stable only at low temperatures,261 the monocarbonyl adducts of Rh2(DPhBz)4448 and Rh2(DPhF)4444,446 are very stable, most likely due to the presence of the amidinate groups which render the dirhodium core more electron-rich than carboxylate groups.
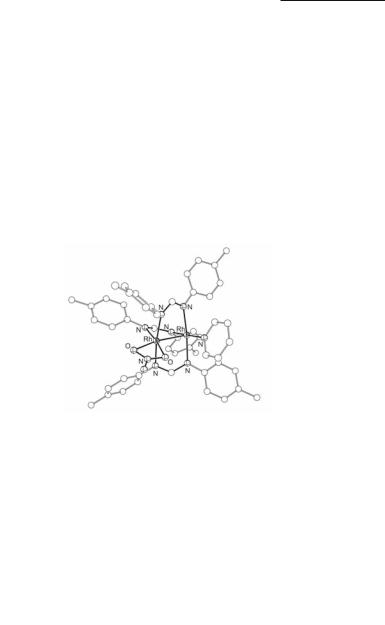
Fig. 12.23. Molecular structure of Rh2(DPhBz)4.
Molecular orbital calculations on the model species Rh2(HNCHNH)4 by the DV-X_ and X_-SW methods revealed that the ground state electronic configuration is μ2/4β2/*4β*2,449,450 which accounts for the strong metal-ligand interactions in the cases of the bridging ligands HNCHNH− and HNNNH−.450 The adduct Rh2(DPhTA)4 with four
Rhodium Compounds 513
Chifotides and Dunbar
1,3-diphenyltriazenide moieties (12.8; Ar = Ph),451 and several compounds with eq molecules of CO and 1,3-di-tolyltriazenide (12.8; Ar = p-tol),452-457 or 1,3-diphenylacetamidinate (12.7; R = CH3, Ar = Ph)458 bridging groups, have been prepared and structurally characterized. In the ‘dimer of dimers’ {[Rh2(DTolTA)2(CO)(bpy)(µ-I)]2}(PF6)2, the two dirhodium units are bridged by two iodide ions. Although the two Rh-I bond distances are not equivalent, they are in the range typical for Rh-halogen bonds encountered in carboxylate compounds.453
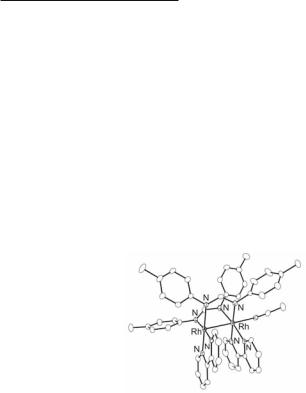
The asymmetric tris-formamidinate complexes Rh2(DTolF)3(δ2-NO3)L (L = PPh3, pyridine, Me2NH)459 are formed by reaction of the paramagnetic Rh25+ complex Rh2(DTolF)3(δ2-NO3)2460 with an excess of the neutral ligand L, via reductive elimination of one nitrate group. The adducts with L = PPh3, pyridine (Fig. 12.24) have L occupying an eq site on one Rh atom and a chelating nitrate group bound to the other rhodium atom.459 The nonequivalence of the two Rh atoms is consistent with the 103Rh NMR spectra of these complexes, each of which shows two well-separated resonances.459
Fig. 12.24. Molecular structure of Rh2(DTolF)3(δ2-NO3)(py).
The mixed bis-trifluoroacetate complex cis-Rh2(DTolF)2(O2CCF3)2(H2O)2445 and the partially solvated cis-[Rh2(DTolF)2(NCCH3)6](BF4)2461,462 are obtained by oxidation of [Rh(COD)(DTolF)]2463 (COD: cycloocta-1,5-diene) with AgO2CCF3 and AgBF4, respectively, according to the reactions:
The ax water molecules of cis-Rh2(DTolF)2(O2CCF3)2(H2O)2 are easily displaced by pyridine, DMSO, piperidine and methylimidazole, without altering the basic structure. The 1:1 adducts with various phosphorus donors PR3, however, give rise to structures with a chelating CF3CO2- group and an eq phosphine on each Rh atom.464 Contrary to the tetraformamidinate compounds which are inert to eq substitution,465 both cis-Rh2(DTolF)2(O2CCF3)2(H2O)2 and cis-[Rh2(DTolF)2(NCCH3)6](BF4)2 exhibit rich chemistry and are useful starting materials due to the increased lability of the ligands trans to the formamidinate groups (the latter exert a strong trans influence).466,467 The polycyano acceptor molecules TCNE, TCNQ, DMDCNQI, DCNNQI,468 and a variety of mesopyridyl-469 and dicarboxylate-470 porphyrins have been reacted with cis-Rh2(DTolF)2(O2CCF3)2(H2O)2 to afford species with notable electro-
514Multiple Bonds Between Metal Atoms Chapter 12
chemical properties. The same starting material has been used to prepare cis-Rh2(DTolF)2- (µ-O2CC6H4CN)2(py)2,471 cis-Rh2(DTolF)2(µ-PPh2Py)2(O2CCF3)2472 with (N, P) donor bridging 2-(diphenylphosphino)pyridine ligands, as well as two orthometalated compounds with only one formamidinate bridging group.465,473 The complex cis-[Rh2(DTolF)2(NCCH3)6](BF4)2 has been employed to prepare the biologically relevant compounds cis-[Rh2(DTolF)2(9-EtAdeH)2- (NCCH3)](BF4)2,462 cis-[Rh2(DTolF)2(9-EtGuaH)2(NCCH3)](BF4)2462 (see Section 12.7.3) and cis-[Rh2(DTolF)2(N-N)n(NCCH3)m]2+, N-N = bpy or phen, n = 1 or 2, m = 1-4.474 A notable aspect of cis-[Rh2(DTolF)2(N-N)n(NCCH3)m]2+ compounds is that the N-N ligands occupy eq-eq sites only474 (e.g., cis-[Rh2(DTolF)2(bpy)2(NCCH3)]2+; Fig. 12.25), unlike dirhodium carboxylate derivatives wherein the N-N ligands may occupy ax-eq351,372 or eq-eq351,373 positions (Figs. 12.16 and 12.17). This behavior is most likely due to the strong trans influence of the formamidinate groups, which render the groups trans to them more labile and thus eq positions readily available to N-N ligands.474
Fig. 12.25. Molecular structure of the cation in cis-[Rh2(DTolF)2(bpy)2(NCCH3)](BF4)2.
The amidinate compounds that have been structurally characterized are listed in Table 12.4. The Rh–Rh distances of the tetraamidinate compounds (2.389-2.570 Å) are longer than those of the carboxylate analogs; this can be partially ascribed to the ‘bite’ of the amidinate groups.299,445 Amidinate, e.g., Rh2(DPhF)4,444 Rh2(DTolF)4,446 Rh2(DPhF-m-OMe)4,442 Rh2(DPhF-3,5-Cl2)4,442 Rh2(DPhBz)4,448 and triazenide (Rh2(DPhTA)4451 complexes lacking ax ligands, as well as others with only a small or linear ax ligand, e.g., Rh2(DPhF)4(NCCH3),444 Rh2(DPhF)4(CNPh),475 Rh2(DPhBz)4(CO),448 [Rh2(DTolF)2(bpy)(NCCH3)3](BF4)2,474 [Rh2- (DTolF)2(phen)(NCCH3)3](BF4)2474 and cis-[Rh2(DTolF)2(9-EtAdeH)2(NCCH3)](BF4)2,461,462 are not uncommon among complexes with (N, N) donor ligands. The absence of ax ligands in the foregoing compounds has been attributed primarily to steric crowding of (N, N) bridging groups as well as to electronic factors.444,446,474,476 The aforementioned reasons are presumably responsible for the scarcity of tetraamidinate Rh24+ units associated in chains by ax ligands, in sharp contrast to tetracarboxylate complexes. Among the rare exceptions are the ‘dimer of dimers’ (DPhF)4Rh2(CNPhNC)Rh2(DPhF)4,475 the ‘trimer of dimers’ {[Rh2(DTolF)4]3(1,4-CNPhNC)2}477 and the polymer [Rh2(DTolF)4(1,4-CNPhNC)] 477 linked by the bidentate di-isocyano ligand 1,4-CNPhNC and the benzene ring acting as an appropriate spacer of the neighboring formamidinate ligands. Conversely, [Rh2(cis-DAniF)2]2+ moieties, with two cisoid formamidinate anions as subunit precursors linked by polyfunctional ligands, e.g., dicarboxylate groups, have been assembled in 1- and 2-D molecular tubes, loops, squares, triangles, double helices and other supramolecular arrays,13,15,469,470,478,483 as well as ‘host’ arrangements capable of encapsulating ‘guest’ molecules of appropriate size484 (Section 12.7.2).
