
Physics of biomolecules and cells
.pdf
198 |
Physics of Bio-Molecules and Cells |
2.4 Uncorrelated failure of bonds loaded in parallel
2.4.1 Markov sequence of random failures
The same series of master equations (2.7) describe the evolution of the n- bonded states for systems loaded in parallel (Fig. 2.1c). However, here force is distributed across the bonds that are intact. As for series loading, the probability of a bond failure increases with the number of intact bonds; but in parallel loading, amplification of unbonding kinetics is reduced due to sharing of the force. Partitioning of force between N bonds is equivalent to an N -fold increase in the thermal force. For bonds in parallel, therefore, the failure rate and e ective thermal force scale for each level are n/to and nfβ respectively, i.e. νn→n−1(f ) = (n/to ) exp[f /(nfβ )]. Even with no rebinding at any step, analytical solution of the master equations is not possible in general for N bonds loaded in parallel so we will employ the equivalent single-bond attachment model to derive an analytical approximation for rupture strength and then compare this prediction to results obtained from numerical computations.
2.4.2 Equivalent single-bond approximation
For uncorrelated failure of bonds in parallel, we assume that force is shared equally amongst existing bonds in the attachment. Thus, the force experienced by bonds increases with each failure event during detachment, i.e. f /bond = f /(N − j) from j = 0 → N − 1. Also, assuming identical bonds, there are N − j possibilities for unbinding at each step, which increases the frequency for bond failure by the factor (N − j). Thus, the e ective rate to detach multiple bonds is estimated by the reciprocal of the times to pass from one state of bonding to the next,
1/νN →(f ) = to Σ1→N (1/n) exp[−f /(nfβ )]. |
(2.11) |
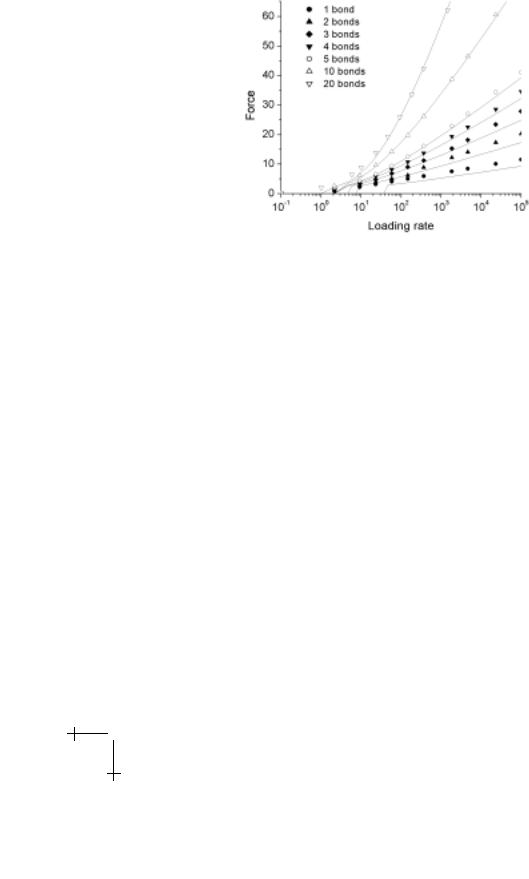
Using this single-bond approximation, equation (2.8) predicts that the force needed to rupture multiple-bonds loaded in parallel is essentially (but not quite) N -fold larger than for a single bond at the same rate of loading (see Fig. 2.6). Beyond the low force range, the strength of a multiple-bond attachment is predicted to follow a transcendental relationship given by,
f ≈ N fβ [log(rf ) − log(f /fβ )] |
(2.12) |
which shows that the most probable rupture force is always somewhat less than N -times the single bond value. It is often assumed that multiple attachments will produce exactly N -fold increase in strength or rupture force, which is only approached in the large N limit. This contradicts the naive interpretation invoked in many early AFM studies to rationalize the
E. Evans and P. Williams : Dynamic Force Spectroscopy |
199 |
Fig. 2.6. Rupture forces (scaled by fβ ) found by solution to the master equations (symbols) for detachment of between 2 and 20 bonds loaded in parallel. (Loading rate is scaled by fβ /to ). The strength of the attachment increases in reasonable proportion to the number of bonds in the attachments. For comparison, the rupture force predicted by the equivalent single-bond attachment approximation (Eq. (2.12)) is plotted as the solid curves, which closely approaches the computational results especially for large numbers of bonds.
apparent presence of a few bonds. Moreover, we will show next that a unitary rupture force per bond cannot be derived from simple analysis of force histograms. The reason is that bond strength is a dynamic property and dependent on the rate of loading. Thus, sharing the load between multiple attachments reduces the single-bond loading rate and hence shifts the force at which bonds break at each step in detachment.
2.5 Poisson statistics and bond formation
The most obvious feature anticipated in tests of multiple bond attachments is that distributions of rupture forces will be very broad. The immediate question that comes to mind is whether or not the spread σN in the force distribution and the mean force fN are related in some well-defined way to the number of bonds N in the attachments. From our equivalent single-bond attachment model, we would expect (Eq. (2.12)) that the mean rupture force will scale with N at very fast loading rates. Moreover, as noted in Part I, the spread in a force distribution for single bond rupture is set by the slope of force versus log(loading rate), i.e. σf fβ . Hence,
200 Physics of Bio-Molecules and Cells
we might naively expect the spread in the force distribution for rupture of multiple bonds loaded in parallel to scale as σN N fβ when N is large. This behaviour implied at large N seems consistent with the perspective of “force quanta”. As such, we might expect to use Poisson statistics to derive a “unitary force” from moments of force distributions for multiple bond detachment. Indeed, such approaches have been proposed and employed in several studies [15–18]. The rationale begins with the view that if the probability of bond formation on contact is low, the number of bonds present in attachments will be sampled from a Poisson distribution. Thus, as predicted by this distribution, the average number of attachments formed in an infinite number of repeated contacts, N , equals the variance in number formed, σN2 . The next – and seriously flawed – assumption is that all forces scale with the number of bonds in an attachment multiplied by the unitary force f1 so that both the average rupture force and variance in force would be, fN = N f1 and σN2 = N f12 respectively. Hence, the unitary rupture force f1 could be found by dividing the variance of a force distribution by the mean, or more robustly, determining the slope of variance versus mean force taken from experiments with a random number of bonds/attachment. However, the assumption that all n-bonded attachments are characterized by a common-unitary force can’t be true given the kinetic dependence of rupture force on loading rate. Even though the mean force for rupture of multiple bonds at fixed loading rate is closely proportional to N at large N , the variance in distributions changes nonlinearly at large N as shown below (and demonstrated qualitatively by the analytical expression for an equivalent single bond, 1/σN2 = νN →[∂2(1/νN →)/∂f 2]f =f ).
To critically test the Poisson hypothesis, we used the master equations to simulate force distributions [pN (f ) = dSu/df ] for rupture of multiple bond attachments and then determined both the mean and variance. In particular, we focussed on a dimensionless rate, rf = rf to /fβ = 1000 because it is comparable to the e ective rates employed in recent AFM studies of mulitiple biotin/streptavidin bonds. This dimensionless loading rate is based on actual loading rates rf of 105 to 106 pN/s and transition state properties of fβ 40 pN and to 0.1 s under fast loading. In performing these computations, the initial populations of bonding states were defined by the Poisson distribution
Sn = exp −N |
N n |
(2.13) |
n! |
where we varied N from 0.1 to 20. As evident in Figure 2.7, variance in force diminishes with increase in the mean force. The derivative of variance with respect to mean force is largest ( 4.6) at low force and is always less than the mean force for single bond rupture ( 6.9). Hence, the rupture

E. Evans and P. Williams : Dynamic Force Spectroscopy |
201 |
Fig. 2.7. Variance in rupture force distributions plotted against the mean as the average number of bonds formed on contact N varies between 0.1 and 20 under the assumption of precise parallel loading. The distributions were obtained by solution to the master equations computed at a dimensionless loading rate of 1000. Clearly, the variance in force does not increase linearly with the mean and demonstrates that a “Poisson-type” analysis cannot be applied to tests of multiple bond attachments. Inset is one of the distributions of rupture forces computed for multiple bonds distributed randomly about an average 20 bonds/attachment where at a rate of 1000, the most probable force for rupture of a single attachment is 6.9.
force for a single bond can only be estimated from extrapolation of the slope of variance versus mean force when the probability of attachment is well below 1. Inset in Figure 2.7 is one of the distributions of rupture forces computed for multiple bonds distributed randomly about an average 20 bonds/attachment. Clearly, the rupture forces in this distribution fall o monotonically away from a single peak with no evidence of quantization or hint of multiple peaks. So we see that the ratio of variance – to – mean of force distributions for large numbers of bonds/attachment cannot provide the force for rupture of a single bond.

202 |
Physics of Bio-Molecules and Cells |
Single molecule experiments are not easy to achieve in practise. To measure the force between two molecules, the likelihood of bond formation on contact has to be reduced to a “limit of dilution” through control of surface chemistry, molecular density, contact force and time. Even when the contact area and surface chemistry are controlled, multiple interactions can still become significant. Unfortunately, as just discussed, there appears to be no simple way to extract precise-quantitative information about a single bond from measurements of multiple bond detachments. However, in this regard, Poisson statistics can provide an important estimate of the likelihood of multiple interactions amongst a series of tests of bond strength. If a low probability of bond formation exists upon contact and we control the mechanics of contact to be equivalent each time, then the fraction of surface contacts that adhere in repeated trials Nf /Nt (number forces against total number of tests) can be used to estimate the mean number of bonds/attachment,
∞ |
|
|
|
|
|
n |
|
Nf |
|
|
|
|
|
N |
|
|
|||
|
exp |
|
|
|
|
|
= |
|
(2.14) |
|
−N |
|
|
|
|||||
n=1 |
|
n! |
Nt |
||||||
|
|
|
|
|
|
|
|
|
|
and therefore the mean number per contact is predicted to be,
|
|
Nt |
|
|
N = ln |
(2.15) |
|||
Nt − Nf |
||||
|
|
|
||
which we can relate to the fraction of single-bond events Ns/Nt in the data,
Nt |
|
Nf |
|
|
Nf |
Nt − Nf |
· |
|
||||||||
Ns |
= |
Nt |
|
|
exp |
|
|
|
= |
Nt − Nf |
ln |
|
Nt |
|
(2.16) |
|
|
|
|
N |
|
N |
|
|
|
|
|
||||||
For example, a 70% frequency of adhesive attachment implies that the average number of bonds formed on each contact is 1.2 and, most important, that nearly half of the forces measured will be from multiple interactions. On the other hand, a 20% frequency of attachment implies that nearly 90% of the forces recorded are due to single rupture events. Hence, our aim in experiments should be to achieve a reduced level adhesion events (around 20%) which always involves the frustrating trade o between likelihood of single events vis a vis the duration of data acquisition. To emphasize again our comments from the previous chapter, each rupture event is a particular sample from a distribution of forces and therefore many hundred forces are needed to describe the process at each rate. With adhesion events only occurring one in every ten or so trials, and with the need to test many orders of magnitude in loading rate, many thousands of tests are required to explore the full kinetic process. This introduces significant challenges to instrument design, requires durable sample preparation, and tests the patience of the investigator!

E. Evans and P. Williams : Dynamic Force Spectroscopy |
203 |
2.6 Summary
We have enlarged the scope for dynamic strength of molecular interactions to include detachment of multiple bonds. We’ve emphasized that whilst experiments may be designed with the utmost care, contacts in experiments can often lead to multiple bond attachments because of the vagaries of molecular scale association. In this context, we’ve identified three prototypical mechanisms for detachment of bonds in attachments: series, parallel, and zipper loading. In nearly all situations of adhesive rupture, multiple bond interactions involve uncorrelated nanoscale excitations and thus the kinetics of failure can be treated as independent random processes with only force related to time and the number of surviving bonds in an attachment. However in the exceptional case of mechanically-constrained multiple bonds, cooperative unbinding can arise and thereby the system behaves as a single “compound” bond. As a rare but significant example, experiments on oligonucleotides have revealed the profound impact of cooperativity, which is to extend the natural lifetime of a complex enormously. Focussing principally on uncorrelated failure of bonds, we first treated a series of bonds that break in random sequence, then a zipper of bonds that break in deterministic sequence but at random times, and finally multiple bonds loaded in parallel that again fail in random sequence but which share an equal force that increments with each unbinding event. To follow the detailed evolution of a collection of bonds, we described how a series of master equations is employed to account for both deterministic and random changes force. But even with no rebinding between bond-breakage steps, analytical solution of the master equations is only possible in special cases. So by comparison to numerical computations of the master equations, we demonstrated that an equivalent single-bond model provides a good approximation for strength of multiple bond attachments. To first order, multiple bond attachments loaded in series or peeled apart like a zipper have strengths comparable to that of a single-constituent bond. For a series of chemically distinct bonds, the most likely site of failure is the “weakest” bond but we showed that “weak” versus “strong” must be viewed as dynamical characteristics. By comparison to systems dominated by single bond strength, multiple bonds loaded in parallel withstand forces nearly proportional to the number but only in the limit of large numbers of bonds and very large forces. Because of the nontrivial relationship between attachment strength and the number of bonds, there is no reliable way to extract characteristics of single bond dynamics from statistics of multiple-bond force measurements when the numbers of bonds vary. Moreover, there’s no reason to expect that detachment of an assembly of many bonds will lead to an identical mechanical loading of each bond. Indeed the opposite should be expected – i.e. a subset of bonds will take the load at each step with the remainder only weakly

204 |
Physics of Bio-Molecules and Cells |
stressed if at all. As the bottom line: the most significant uncertainty in interpreting rupture force for attachments with multiple bonds is knowing which of these scenarios most closely represents the nanoscale mechanics of detachment.
The authors gratefully acknowledge support from US National Institutes of Health HL65333, HL31579, Medical Research Council of Canada grant MT477 to (EE) and the Engineering and Physical Sciences Research Council, the Biological and Biomedical Sciences Research Council, Universitas 21 and the University of Nottingham to (PMW).
References
[1]E. Evans and K. Ritchie, Biophys. J. 72 (1997) 1451.
[2]U. Dammer, et al., Biophys. J. 70 (1996) 2437.
[3]S. Allen, et al., FEBS Lett. 390 (1996) 161.
[4]J.M. Williams, T.J. Han and T.P. Beebe, Langmuir 12 (1996) 1291.
[5]E. Evans, Faraday Discuss. Chem. Soc. 111 (1998) 1.
[6]R. Merkel, et al., Nature 397 (1999) 50.
[7]E. Evans, et al., Proc. Natl, Acad, Sci, USA 98 (2001) 3784.
[8]E. Evans, Annu. Rev. Biophys. Biomol. Struct. 30 (2001) 105.
[9]T. Strunz, K. Oroszlan, R. Shafer and H.-J. Guntherodt, Proc. Natl. Acad. Sci. USA 96 (1999) 11277.
[10]L. H. Pope, et al., Eur. Biophys. J. Biophys. Lett. 30 (2001) 53.
[11]M. Rief, et al., Science 276 (1997) 1109.
[12]E. Evans and K. Ritchie, Biophys. J. 76 (1999) 2439.
[13]C. Gergely, et al., Proc. Natl. Acad. Sci. USA 97 (2000) 10802.
[14]H. Skulason and C.D. Frisbie, J. Amer. Chem. Soc. 112 (2000) 9750.
[15]F. Stevens, Y.-S. Lo, J.M. Harris and T.P. Beebe, Lanmguir 15 (1999) 207.
[16]Y.-S. Lo, et al., Langmuir 15 (1999) 1373.
[17]Z.Q. Wei, et al., Surf. Sci. 459 (2000) 401.
[18]Y.-S. Lo, Y.J. Zhu and T.P. Beebe, Langmuir 17 (2001) 3741.

SEMINAR 1
POLYMERIZATION FORCES
M. DOGTEROM
FOM Institute AMOLF,
Kruislaan 407, 1098 SJ Amsterdam,
The Netherlands

POLYMERIZATION FORCES
M. Dogterom
The mechanical framework (cytoskeleton) of higher order (eukaryotic) cells consists of three types of protein filaments: actin filaments, intermediate filaments, and microtubules [1]. The assembly or polymerization of both actin filaments and microtubules has been implicated in cellular force generation processes. Examples are: the pushing forward of membranes by polymerizing actin filaments in the leading edge of crawling cells [2]; the propulsion of Listeria bacteria through their host cell, again by polymerization of actin filaments [3,4]; and the motion of chromosomes by the assembly and disassembly of microtubules during the process of cell division [5]. In this seminar I will describe how force is expected to a ect the assembly of cytoskeletal filaments [6–8] and describe experiments designed to measure the forces generated by single growing microtubules.
Let us first consider the assembly of a single linear filament [9]. Above the so-called critical subunit concentration, the (concentration-dependent) rate at which subunits attach (the on-rate) is higher then the (concentrationindependent) rate at which they detach (the o -rate). The velocity of polymerization is positive and given by:
V = δ(kon − ko ) |
(1) |
where δ is the subunit size.
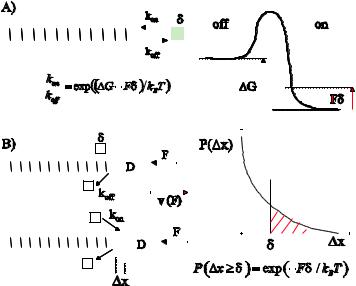
Figure 1A shows schematically the free energy di erence ∆G between the on-and o -states of a subunit as well as the activation barrier for transitions between the two. The absolute values of the onand o -rates depend on the activation energy, but the ratio kon/ko does not. In the absence of load this ratio is simply given by exp(∆G/kBT ), where kB is Boltzmann’s constant and T is the absolute temperature. When a load is applied, the
This work is part of the research program of the “Stichting voor Fundamenteel Onderzoek der Materie (FOM)”, which is financially supported by the “Nederlandse organisatie voor Wetenschappelijk Onderzoek (NWO)”.
c EDP Sciences, Springer-Verlag 2002
208 |
|
|
|
|
|
Physics of Bio-Molecules and Cells |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 1. Polymerization of a single filament in the presence of a load (see also text). A) Thermodynamic arguments predict that the ratio between the onand o -rate decreases exponentially with an applied force. The gain in free energy, ∆G, associated with the assembly reaction is reduced by Fδ, the amount of work performed per assembling subunit. B) Alternatively, the assembly process can be viewed as a thermal ratchet, where the on-rate is reduced by the probability P (∆x ≥ δ) that thermal fluctuations create a gap between the filament and a barrier large enough to insert a new subunit. D is the di usion constant of the barrier.
gain in free energy due to assembly of a subunit is reduced by the amount of work performed to ∆G-Fδ, which gives:
kon(F ) |
= e(∆G−F δ)/kBT = |
kon (0) |
e−F δ/kBT . |
(2) |
ko (F ) |
|
|||
|
ko (0) |
|
||
This formula gives the relative e ect of force on the onand o -rates, but not the absolute e ect. To obtain the latter, the activation energy is needed. The growth velocity of the filament as a function of force (the force-velocity curve) is given by:
V (F ) = δ kone−qF δ/kBT − ko e(1−q)F δ/kBT |
(3) |
where the value of q depends on how much the o -rate is a ected by the force relative to the on-rate. The force needed to stall the assembly of the
