
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
490 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
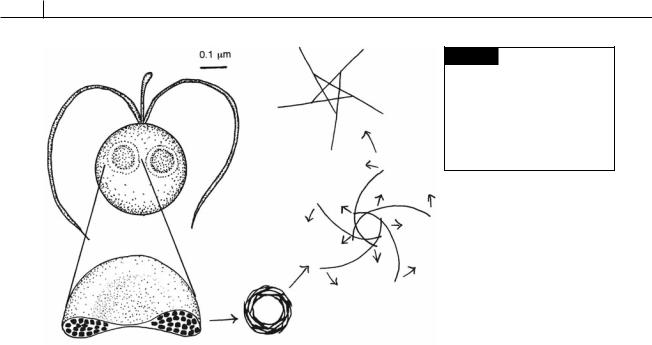
Fig. 22.9 Phaeocystis globosa showing a cell with two vesicles containing coiled filaments made of chitin. The coiled filaments unwind when they are discharged outside the cell, forming a 5-ray star structure. (After Chrétiennot-Dinet et al., 1997.)
The symbionts are held in the rhizopodial network surrounding the central capsule of the radiolarian. The algal symbionts fix carbon dioxide, and some of the photosynthate passes to the radiolarian.
Scales and coccoliths
Most of the members of the Prymnesiophyceae have a cell covering consisting of a number of elliptical scales (Figs. 22.1, 22.10). The elliptical scales are embedded in a mucilaginous substance, and in some organisms a layer of calcified coccoliths is outside the scales. The structure of the scales can vary considerably, but all of them appear to have an elliptical organic scale constituting either the whole structure of the scale or just the base plate on which the rest of the structure is based. The elliptical organic scale (plate scale) has radiating ridges extending to the edge (Fig. 22.10(a)) (Parke and Manton, 1962). Chrysochromulina minor (Fig. 22.10(b)) (Parke et al., 1955) and Chrysochromulina parva (Fig. 22.6(b)) (Parke et al., 1962) have a cell covering of only plate scales of the type just described. In other Prymnesiophyceae the rims of the elliptical base plate have become turned up to form a variety of different types of scales ranging from
very shallow tub-shaped scales with the edges of the scales barely turned up, to very long spined structures with the spine being the very elongated upturned rim of the organic scale (Fig. 22.10(c)) (Manton, 1972). A number of different types of scales can be present on the same organism. Chrysochromulina kappa (Fig. 22.10(d)) has plate scales around most of the body with a few short-spined scales near the flagella (Parke et al., 1955). Chrysochromulina ericina (Fig. 22.10(e)) has a cell covering consisting of plate scales with about 30 long-spined scales intermixed with the plate scales (Parke et al., 1956). Chrysochromulina pringsheimii (Fig. 22.10(f )) has an even more complex cell covering consisting of four types of scales. Long-spined scales are at either end of the cell with small-spined scales covering the rest of the cell. Underneath this spined layer is a layer of plate scales, and, finally, near the base of the flagella are a number of small plate scales (Parke and Manton, 1962).
The organic scales originate in the Golgi apparatus (Figs. 22.1, 22.13) (Hawkins and Lee, 2001). A scale, when first formed in a Golgi vesicle, is closely enveloped by the vesicle membrane no matter how elaborate the shape of the scale is (long-spined, etc.); but immediately before the scale is liberated to the outside of the cell, this close contact is lost (Manton, 1967b).

PRYMNESIOPHYTA 491
Fig. 22.10 (a) Small plate scales of Chrysochromulina pringsheimii, the upper face on the left and the lower face on the right.
(b) Chrysochromulina minor, individual swimming with the flagella and haptonema behind the body in the position characteristic of the species during rigid swimming.
(c) Chrysochromulina parkae, two forms. (d) Chrysochromulina kappa, swimming with the flagella and haptonema behind the body, the characteristic position of species during rapid swimming.
(e) Chrysochromulina ericina, individual with dividing chloroplasts anchored by a haptonema which is partially extended; the flagella are in the characteristic position when the cells are stationary.
(f) Chrysochromulina pringsheimii, individual swimming with the flagella and haptonema in front of the body, and the haptonema fully extended.
(c) Chloroplast; (f) flagellum;
(h) haptonema; (l) leucosin vesicle; (lss) long-spined scale;
(m) muciferous body; (n) nucleus;
(p) pyrenoid; (ps) plate scale;
(s) scale; (sp) spine; (ss) spined scale; (sss) small-spined scale. ((a), (f) after Parke and Manton, 1962; (b), (d) after Parke et al., 1955; (c) after Green and Leadbeater, 1972; (e) after Parke et al., 1956.)
There is a diurnal rhythm in the production of scales. Manton and Parke (1962) showed that in
Chrysochromulina polylepis the greatest production of scales is in the late afternoon with the least in the early morning hours. The time of nuclear division is the reverse, with the most mitotic figures appearing in the early morning.
Even in the non-motile filamentous stages of the Prymnesiophyceae, the cell wall is composed
of scales embedded in a gelatinous matrix. In
Pleurochrysis scherffelii (Fig. 22.11) the cells of the filamentous stage are covered in cellulosic scales produced by the single Golgi apparatus. By the use of the cinephotography, Brown (1969) showed that during wall formation the whole protoplast revolves so that the scale secretions of the Golgi apparatus are received more or less evenly by all portions of the cell wall.

492 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
(a)
(b)
Fig. 22.11 Pleurochrysis scherffelii. (a) Motile cell.
(b) Filamentous stage. (After Pringsheim, 1955.)
Coccoliths are calcified scales of the Prymnesiophyceae. They were originally described as minute carbonate discs in Cretaceous deposits and thought to be of inorganic origin (Marsh, 2004). Later they were in sea bottom oozes brought up by the first Atlantic cable survey in 1858. Their algal nature was not recognized until 1898. Coccoliths are basically organic scales that have calcium carbonate (CaCO3) deposited on one surface in a characteristic pattern depending on the species of the alga. Under ordinary conditions anhydrous CaCO3 exists in nature in two crystalline forms (Fig. 4.7), calcite (rhombohedral) and aragonite (orthorhombic), which differ in structure, hardness, specific gravity, and solubility. In coccoliths the form of CaCO3 is usually calcite. The calcite is attached to the outer surface of a plate scale, which has a pattern of radiating ridges (Figs. 22.13, 22.24) whereas the inner side of the scale (toward the cell) is virtually patternless. The coccoliths of Emiliania huxleyi (Fig. 22.16(b)) are relatively simple and can serve as an example of coccolith structure although it should be realized that there is a multitude of different coccolith forms, some very complex (Fig. 22.16). The coccoliths of E. huxleyi (Figs. 22.12, 22.16(b)) are composed of a number of hollow crystals of calcite arranged around the periphery of a plate scale (Young et al., 1992). Each coccolith consists of an upper and lower shield joined by a tube. The tube and the shields are composed of subunits.
Coccolith formation begins with the production of an uncalcified organic plate-scale in the
center of Golgi cisternae (Figs. 22.13, 22.14) (Marsh, 1996, 2004). Highly acidic polysaccharides are produced as 25 nm diameter spheres in the peripheral part of Golgi cisternae. The highly acidic polysaccharides are extremely negative polyanions that sequester large numbers of calcium ions. The 25 nm diameter spheres are pinched off the Golgi cisternae as vesicles, which eventually fuse with a vesicle containing an uncalcified organic plate-scale. The 25 nm diameter spheres aggregate on the scales in the area of future calcium deposition. Calcium is deposited in the area of the 25 nm diameter spheres to produce calcified coccoliths. The remaining material in the spheres is reorganized into an amorphous coat that surrounds the mature crystals of calcium carbonate that make up the coccoliths. The coccoliths are carried in the vesicles to the plasma membrane where the coccoliths are deposited outside the cell. Two to seven coccoliths are produced per day by Emiliania huxleyi (Balch et al., 1993; Linschooten et al., 1991).
Coccoliths are detached from cells in layers at the same time as other coccoliths are produced. During logarithmic growth, about the same number of coccoliths are detached as are produced. In stationary growth, however, the rate of coccolith detachment increases about threefold, while coccolith production drops off (Balch et al., 1993). Coccolithophorids have greater rates of calcification when nitrogen and phosphorus are limiting in the seawater (Corstjens and Gonzales, 2004).

|
|
|
|
|
|
PRYMNESIOPHYTA |
493 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Upper Shield |
|
|
|
Fig. 22.12 |
Construction of a |
|
||
|
|
Elements |
|
|
|
|
||
|
|
|
|
|
heterococcolith of Emiliania huxleyi. |
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
The heterococcolith is composed of |
|
|
|
|
|
|
Tube |
|
a tube joining the upper and lower |
|
|
|
|
|
|
Elements |
|
|
||
|
|
|
|
|
shields, each of which is composed |
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
of subunits. (Adapted from Young |
|
|
|
|
|
|
|
|
et al., 1992.) |
|
|
|
Lower Shield |
|
|
|
|
|||
|
|
|
|
|
|
|
||
|
Elements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 22.13 Coccolith formation in Pleurochrysis. Spheres containing calcium bound to acidic polysaccharides are produced in vesicles by the Golgi apparatus. These vesicles fuse with Golgi vesicles containing uncalcified organic scales. The calcium-containing spheres become positioned on the scales at the sites of calcification and the calcium is released. The calcified scales are carried to the plasma membrane, releasing the coccoliths outside of the cell. (Adapted from Marsh, 1994.)
Coccoliths can have an outer covering of heterococcoliths that contain assemblages of morphologically complex and diverse CaCO3 elements (Figs. 22.12, 22.14, 22.16), which are composed of similar (often rhombohedral) calcite crystals (Fig. 22.15). The two types of coccoliths can occur in the same organism with heterococcoliths on the
diploid cells and holococcoliths on the haploid cells (Geisen et al., 2002). In Coccolithus, holococcoliths occur in the motile Crystallolithus stage (Fig. 22.15) whereas heterococcoliths occur in the non-motile stage. The base plates of heterococcoliths are produced in Golgi cisternae followed by calcification in the same cisternae. However, only the base plates of holococcoliths are produced in Golgi cisternae. These base plates are discharged outside the cell where calcification occurs within an outer vestment (Rowson et al., 1986).
The coccolithophorids (algae with coccoliths) (Fig. 22.16) are common in tropical waters because these warm waters have a low partial pressure of carbon dioxide and are usually saturated or supersaturated with calcium carbonate,

494 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 22.14 Top: Intact heterococcolith of Hymenomonas carterae. Bottom: The calcium carbonate making up the heterococcolith is partially dissolved away, revealing the scale on which the calcium carbonate is deposited. (From Outka and Williams, 1971.)
the concentrations being especially high in the upper layers. Supersaturation of calcium carbonate is favorable for the formation of coccoliths, and the distribution of coccolithophorids shows a close correlation with the degree of saturation of seawater by calcium carbonate. In the seas of polar regions, the degree of saturation does not even reach 90%.
The calcification reaction is:
|
Ca |
2 |
CaCO3 |
CO2 H2O |
2 HCO3 |
|
Carbon dioxide is released in calcification, and this may make coccolithophorids more competitive by increasing the amount of CO2 available for photosynthesis inside the cell (Nielsen, 1995). This may be particularly important in seawater where the pH of 8.2 results in a very low concentration of dissolved CO2 of about 10 M compared to a total dissolved carbonate (mostly HCO 3) of 2000 M.
The membrane of the coccolith vesicle contains an H2 ATPase that pumps H out of the coccolith vesicle, removing protons produced during dehydration of bicarbonate and increasing the rate of calcification (Corstjens and Gonzales, 2004; Gonzales, 2004).
Fig. 22.15 Scanning electron micrograph of holococcoliths covering a cell of Crystallolithus hyalinus. (From Faber and Preisig, 1994.)

PRYMNESIOPHYTA 495
Fig. 22.16 Scanning electron micrographs of coccolithophorid cells. (a) Coccolithus pelagicus. (b) Emiliania huxleyi. (c) Discosphaera tubifera. (d) Pontosphaera syracusana.
(e) Syracosphaera nodosa. (f) Braarudosphaera bigelowii. (From Faber and Preisig, 1994.)
