
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
HETEROKONTOPHYTA, CHRYSOPHYCEAE |
337 |
|
|
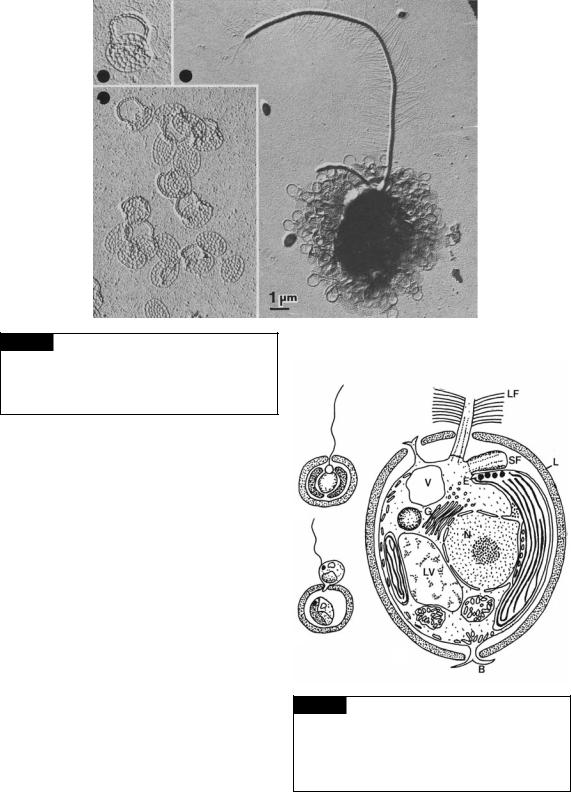
Fig. 10.4 Transmission electron micrographs of
Paraphysomonas sigillifera showing a whole cell with a long tinsel flagellum and a short whiplash flagellum. Also included are higher-magnification micrographs of the scales. (From Thomsen et al., 1981.)
Chrysococcus rufescens (Fig. 10.5). This alga has a basically mucilaginous lorica surrounding the cell (Belcher, 1969). In young cells the lorica is very transparent, probably consisting just of mucilage. At a later stage, dark needle-shaped crystals appear, which are primarily oriented parallel to the cell surface. The crystals ultimately unite to form a felty mass outside the cell.
Anthophysa vegetans (Fig. 10.10(b), (c)) is a freshwater colonial alga that produces a mineralized stalk that can contain salts of calcium, iron, or manganese. The minerals in the environment determine color and the mineral composition of the stalk (Lee and Kugrens, 1989).
(a)
(b)
(c)
Statospores
The formation of a cyst or statospore or resting spore is one character by which a member of the Chrysophyceae or Synurophyceae may be unequivocally recognized. Statospores are mostly
Fig. 10.5 Chrysococcus rufescens. (a) Whole cell. (b) Cell undergoing reproduction. (c) Ultrastructure of vegetative cell. (B) Branched cytoplasmic process; (E) eyespot;
(G) Golgi; (L) lorica; (LV) leucosin vesicle; (LF) long flagellum;
(N) nucleus; (SF) short flagellum; (V) contractile vacuole. ((c) after Belcher, 1969.)

338 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 10.6 Statospore of
Ochromonas sphaerocystis.
Bar 5 m. (From Andersen,
1982.)
Fig. 10.7 Formation of a statospore or cyst in Ochromonas tuberculata. (a)–(c) The formation of the statospore. (d)
A mature statospore as seen from the collar end. (C) Chloroplast; (Co) collar of statospore; (Cr) chrysolaminarin vesicle; (CV) contractile vacuole; (D) discobolocyst; (N) nucleus; (P) plug in pore of statospore; (S) statospore wall; (SDV) silica deposition vesicle; (Sp) spine. (Adapted from Hibberd, 1977.)
spherical, ellipsoidal, or ovate in shape, and the outer surface may be smooth or variously ornamented with warts, spines, or arms (Figs. 10.6, 10.7). Wall ornamentation is species specific. The statospore has a pore with a collar that is closed by a plug. A vegetative cell forms a statospore internally. In the formation of a statospore the cells become non-motile, any projectiles are discharged, and there is considerable contractile vacuole activity (Sheath et al., 1975; Hibberd, 1977). A nearly spherical vesicle called the silica deposition vesicle is formed in the cytoplasm, and silica is deposited
inside the vesicle (Fig. 10.7) (Preisig, 1994). A complete sphere of silica is formed, interrupted only by the developing pore and collar. The nucleus, chloroplast, flagellar basal bodies, mitochondria, Golgi body, chrysolaminarin vesicle, and ribosomes are segregated to the inside of the silica deposition vesicle, whereas outside there are mitochondria, ribosomes, contractile vacuoles, and small vesicles. After the spines, pore, and collar have been formed in the silica deposition vesicle, a plug is formed by the cytoplasm in the pore area. With the formation of the unsilicified plug, contact is lost between the protoplasm inside the statospore and that outside, with the inner membrane of the silica deposition vesicle becoming the new plasmalemma. When a statospore germinates, there is a dissolution of the plug or separation of it from the spore wall. The protoplast then moves out of the statospore by amoeboid motion, forming flagella as it moves out.

HETEROKONTOPHYTA, CHRYSOPHYCEAE |
339 |
|
|
|
|
|
|
|
|
|
|
|
(a) |
|
(b) |
|
(c) |
|
(d) |
|
(e) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 10.8 Phagotrophy in Epipyxia pulchra. The cell has a posterior stalk by which it is attached to a lorica. (a) The long tinsel flagellum beats in such a way that water and suspended particles are drawn to the cell. (b) A particle is seized by the long tinsel flagellum. (c) The particle is maneuvered between the two flagella. (d) A feeding cap from the cell envelopes the particle. (e) The particle is enclosed within a food vacuole within the cytoplasm. The stalk has pulled the cell into the lorica. (Modified from Wetherbee and Andersen, 1992.)
Nutrition
Nutrition in the Chrysophyceae can be either phototrophic, phagotrophic, or mixotrophic (photosynthetic organism capable of taking up particles and molecules from the medium) (Zhang and Watanabe, 2001). Dinobryon (Fig. 10.11) is a mixotrophic chrysophyte that can have half of the carbon in the cells derived from ingestion of microorganisms (Caron et al., 1993). Dinobryon has the ability to compete with crustaceans, rotifers, and ciliates in capturing microorganisms. Under low-light conditions, Dinobryon can ingest an average of three bacterial cells every five minutes (Bird and Kalff, 1986). Chrysophytes have the ability to ingest prey up to 30 times larger than themselves. The ability to take up microorganisms appears to be about the same for pigmented and nonpigmented chrysophytes (Zhang et al., 1996).
Like other mixotrophic chrysophytes, Epipyxis pulchra phagocytizes food particles that are both
living (bacteria, small algae, or even cells of its own kind) and non-living (detritus, fecal material) (Wetherbee and Andersen, 1992). During prey gathering, the long flagellum, which is adorned with stiff hairs, beats rapidly to direct a strong current towards the cell while the short, smooth flagellum moves very little. When a potential food particle is drawn by current to contact the flagellar surface, the long flagellum stops beating and positions itself, in concert with the short flagellum, to seize the prey between the two flagella (Fig. 10.8). Both flagella briefly rotate the prey before selecting or rejecting it. If selected as food, the prey is held in place until a complex collecting cup emanates out and engulfs the prey. The cup plus the prey, now a food vacuole, is then retracted back into the cell proper.
Isofloridoside, a product of photosynthesis in Ochromonas malhamensis, is used to adapt the cells to changes in the dissolved substances (osmotic pressure) of the medium. As the osmotic pressure of the medium increases, O. malhamensis responds by increasing the concentration of isofloridoside inside the cell, preventing water loss to the medium (Kauss, 1967).
Ecology
Fauré-Fremiet (1950) has shown that Chromulina psammobia moves up to the surface of mud flats at

340 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
low tide, and as the tide comes in, the flagellate moves down in the mud so as not to be washed away. The basis for the migration is a reversal in the phototactic response, the cells being positively phototactic at low tide and negatively phototactic at high tide. The presence of mud is not necessary for the changes in phototaxis, which will continue for 6 to 7 days in the laboratory, more or less synchronized with the tide. After this the phototaxis changes gradually until the cells are always positively phototactic.
Dinobryon (Fig. 10.11) is a freshwater alga that is almost never found in waters with a high concentration of phosphorus. This observation led some investigators to conclude that high concentrations of phosphorus are inhibitory to growth of the alga. More detailed work (Lehman, 1976) showed this not to be true, that Dinobryon in culture grows well at high concentrations of phosphorus. What happens in nature is that other algae grow faster and outcompete Dinobryon at higher concentrations of phosphorus; only when vernal (spring) blooms of diatoms or other phytoplankton have reduced phosphorus to a level that inhibits their own growth will Dinobryon compete effectively. This demonstrates a basic rule among algae (Eppley et al., 1969; Fuhs et al., 1972), which is algae that have efficient uptake of nutrients (are able to utilize low levels of a nutrient) usually have lower maximum intrinsic growth rates than algae that are less efficient in taking up nutrients. These differences represent two variations of an adaptive scheme for nutrient utilization. Some species exploit a resource-laden environment rapidly, whereas others display measured efficiency in utilizing an energy source. Energy trade-offs among competing intracellular processes might preclude organisms from excelling at both simultaneously.
Dinobryon uses phosphate not only as inorganic phosphate but also as organically bound phosphate in moderate-sized molecules (glycerophosphate, uridylic acid, adenylic acid) (Lehman, 1976). It can also use nitrogen either from inorganic sources or from organic molecules (urea, glycine, uridylic acid, adenylic acid). Its capacity to utilize these organic sources and its phagocytosis of whole particles imply that Dinobryon is suited to occupy the water column when death and decline of previous bloom-forming organisms release products of cell
breakdown to solution. It is also at these times, when ambient levels of dissolved inorganic nutrients have become somewhat depleted, that the potentials of the cells for effective uptake of nutrients show their advantage.
Although high concentrations of phosphorus do not inhibit the growth of Dinobryon, high concentrations of potassium do (Lehman, 1976). The concentrations necessary for inhibition are commonly found in many lakes. As an example, in one Swedish lake, D. sertularia appears in June a few weeks after the ice breaks, with the alga persisting in the cool water until early autumn (Willén, 1961). Its period of abundance matches exactly with a summertime decrease in potassium caused partly by the precipitation of potassium by clay colloids and partly by the uptake by littoral vegetation (Ahl, 1966). The disappearance of Dinobryon corresponds to increased concentration of potassium as the element is released from dying vegetation.
Chrysophytes are notorious for their production of fishy or rancid smells, reflecting release of unsaturated aldehydes derived from the high cell content of polyunsaturated acids. Uroglena (Fig. 10.10(a)) and Dinobryon (Fig. 10.11) produce unsaturated fatty-acid derivatives (Fig. 10.9) that contribute to these smells (Watson and Satchwill, 2003). These chemicals are classified as algal volatile organic compounds (AVOCs).
Fig. 10.9 Three unsaturated fatty-acid derivatives produced
by chrysophytes that result in rancid or fishy odors.
