
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index

DINOPHYTA 291
Fig. 7.46 Diagram showing how a single endogenous clock within a Lingulodinium polyedrum cell can control a multiplicity of observed rhythms. (After Johnson and Hastings, 1986.)
system that delays the timing, and a blue-light sensitive system that advances the timing to dawn (Roenneberg and Deng, 1997). These light systems probably stimulate or depress the production of melatonin in the cells. An increase in the concentration of melatonin appears to mark the end of the light phase. There is a circadian rhythm in the production of melatonin, with a rapid increase in melatonin concentration at the end of the light phase and a subsequent decline during the dark phase, reaching a minimal value in the light phase. The concentration of melatonin in dinoflagellate cells is similar to that in the mammalian pineal gland (Balzer and Hardeland, 1996). Melatonin may represent a common basis for photoperiodism in organisms as distant as dinoflagellates and vertebrates, a fact that suggests an ancient mechanism for mediating information about darkness within the circadian cycle (Pöggeler et al., 1991).
Heterotrophic dinoflagellates
An estimated half of the more than 2000 living dinoflagellate species lack chloroplasts and are exclusively heterotrophic (Hansen and Calado, 1999; Jacobsen, 1999). In addition, many dinoflagellates that contain chloroplasts are capable of mixotrophy where a portion of their nutrients is obtained heterotrophically, particularly when nutrient conditions are low in the environment (Smalley et al., 2003).
The different modes of heterotrophy are: (1) phagotrophy through the direct engulfment of prey; (2) pallium feeding where the prey is engulfed by a cytoplasmic veil – the palium – with digestion of the food taking place outside of the dinoflagellate cell; (3) peduncle feeding (myzocytosis) involving the uptake of intracellular material of the prey through a cytoplasmic extension – the peduncle – leaving the plasma membrane and extracellular material of the prey behind; and (4) osmotrophy or the uptake of dissolved substances (Höhfeld and Melkonian, 1998).
Direct engulfment of prey
The large cells of Noctiluca have a 300- m-long food-gathering tentacle that is covered with a slimy exudate (Fig. 7.47) (Sweeney, 1978; Nawata and Sibaoka, 1983). Two wing-like extensions of the cells form an oral pouch at the base of the tentacle. At the bottom of the oral pouch is a cytosome that opens like a slit during ingestion of food organisms. Collision of other organisms in the medium with the mucus-covered tentacle tip results in attachment of the other organisms to the tentacle. Continued collisions result in the formation of a clump of cells adhering to the tentacle. The tentacle is primarily in an extended configuration during this time. The tentacle now bends so that the tentacle tip moves to the bottom of the oral pouch. The cytoplasm streams vigorously toward the cytosome and then aggregates around the cytosome (Nawata and Sibaoka, 1987). The cytosome opens, the tentacle tip is inserted into it, the food organisms are swept into the cytosome, and the tentacle tip is retracted. The cytosome opening closes, and the food organisms are engulfed in a food vesicle in

292 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
(a) |
|
(b) |
|
(c) |
|
|
|
|
|
Fig. 7.47 Drawing of the ingestion of food organisms (other algae, bacteria) by Noctiluca. (a) The tentacle (T) is in an extended configuration. Any food organisms (FO) that collide with the mucus-covered tentacle tip, stick to the tentacle. (N) Nucleus; (OP) oral pouch. (b) The tentacle bends back toward the oral pouch. (c) The cytosome (C) at the base of the oral pouch opens, the tentacle tip is inserted into the cytosome, and the food organisms are swept into a food vacuole. (After Nawata and Sibaoka, 1983.)
the cytoplasm. The food organisms are digested in the food vesicle. In the cell under the microscope, long strands of mucus containing food organisms can be seen spirally coiled in the food vacuoles.
Polykrikos kofoides feeds by initially spearing its prey with a nematocyst (Figs. 7.48, 7.49(a), (b)). The prey organism is pulled into the posterior sulcus, with the prey eventually being completely engulfed into a food vacuole.
Pallium feeding
This occurs only in thecate species and utilizes a feeding veil, the pallium, which emerges from the flagellar pore and encloses the prey. The prey protoplasm is digested by enzymes released into the pallium, and the digestion products are transported into the feeding cell. Protoperidinium (Fig. 7.50) and Diplopsalis (Naustvoll, 1998) feed in this manner. These dinoflagellates swim in a straight line until they encounter a prey organism. The dinoflagellate then changes swimming behavior by slowing down and swimming in tight circles around the prey for less than one minute. A thin filament of cytoplasm (about 1 m in diameter) emerges from the sulcal pore and attaches to the prey (Fig. 7.50). A pseudopod is extended along the filament while the filament is retracted, pulling the prey closer to the dinoflagellate. The pseudopod advances at 2 to 6 m s 1 until it reaches the prey. The pseudopod conforms to projections,
Fig. 7.48 Polykrikos kofoides captures prey by firing a nematocyst into the prey and bringing the tethered prey into the posterior portion of the cell. The prey is engulfed and digested by the dinoflagellate. (Modified from Matsuoka et al., 2000.)

DINOPHYTA 293
Fig. 7.49 (a),(b) Scanning electron micrographs of 8-cell pseudocolonies of Polykrikos schwartzii. Ventral view (a) and apical view (b) showing the apical groove. (c),(d) Scanning electron micrographs of Pyrocystis lunula (c) and Pyrocystis noctiluca (d). Pyrocystis is unusual among dinoflagellates in existing primarily in the non-motile vegetative state. Pyrocystis is a major source of bioluminescence in the ocean. ((a),(b) from Nagai et al., 2002; (c),(d) from Seo and Fritz, 2002.)
(a) |
(b) |
(c)
Fig. 7.50 The heterotrophic dinoflagellate Protoperidinium conicum feeding on the diatom Corethron hystria. Initially the dinoflagellate attaches to the prey by a long thin filament (a). Next a pseudopod extends along the filament (b) and engulfs the prey (c), which is digested. (After Jacobsen and Anderson, 1986.)
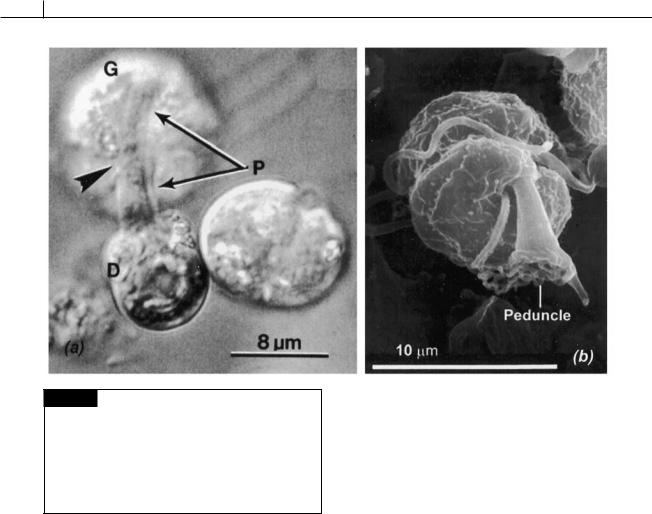
294 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.51 (a) Light micrograph of the dinoflagellate
Gymnodinium fungiforme (G) ingesting the protoplasm of
Dunaliella salina (D). The peduncle (P) of G. fungiforme has attached to D. salina with the protoplasm of D. salina passing through the enlarged and extended peduncle into the dinoflagellate. (b) Scanning electron micrograph of a zoospore of Pfiesteria pisciicida showing the peduncle. ((a) from Spero, 1982; (b) from Lewitus et al., 1999.)
such as spines, and can engulf an organism many times larger than the dinoflagellate. The prey is digested in the pseudopod, with the pseudopod showing active cytoplasmic streaming at a velocity of about 5 m s 1. A diatom is digested by the dinoflagellate in about 30 minutes, at which time the pseudopod is rapidly retracted (at a rate of 10m s 1) into the dinoflagellate.
Peduncle feeding
Gymnodinium fungiforme contains an extensible peduncle (Lee, 1977), a projection of cytoplasm full of microtubules, in the epicone just above the intersection of the sulcus and cingulum (Figs. 7.51(a), 7.52) (Spero and Moree, 1981). The peduncle can extend from 8 to 12 m to attach to, and make a hole into, the prey (Spero, 1982). The cytoplasm of the prey moves through the peduncle to the dinoflagellate cytoplasm. After feeding
is complete, the microtubules of the peduncle, and the peduncle itself, retract back into the dinoflagellate cytoplasm. Feeding is characterized by the aggregation of numbers of the dinoflagellate on the prey organism. A small green alga, such as Dunaliella, has five to ten dinoflagellates attached by their peduncles, whereas a ciliate may have 400 to 500 dinoflagellates attached to it. The peduncle of G. fungiforme apparently cannot penetrate a cell wall, so the dinoflagellate feeds only on prey that lacks a cell wall, or on injured higher organisms. The marine ciliate Condylostoma magnum is not fed on by G. fungiforme until it is injured (in the laboratory by piercing it with a micropipette). Within 15 seconds, more than 100 dinoflagellates will congregate around the wound. When a ciliate is taken from a dying culture and placed in a G. fungiforme culture, the dinoflagellate cells congregate around the posterior end of the ciliate. Initially small strands of ciliate cytoplasm are pulled off by groups of one to five dinoflagellates. As the ciliate begins to leak cytoplasm through these small wounds, large aggregations of phagotrophic dinoflagellates are formed. Approximately 20 to 30 minutes later, the cilate is completely digested. The attacking cells are small (9 m long and 6 m wide), clear cells
