
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index

284 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
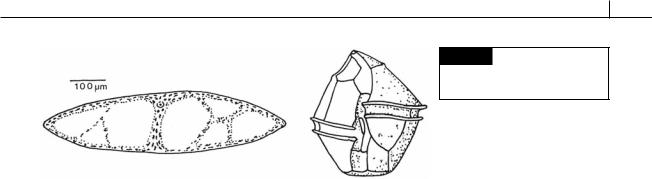
Fig. 7.36 Hypnospores (resting spores) of Alexandrium. (a) Light micrograph of a hypnospore surrounded by mucus.
(b) Scanning electron micrograph showing the smooth surface of a hypnospore. (c) Transmission electron micrograph showing the large amount of storage products in a hypnospore. (d) Transmission electron micrograph of the layers of the cell wall. (e) Illustration of the layers of the cell wall. (cm) Cell membrane. (From Kennaway and Lewis, 2004.)
preceding blooms of diatoms may impoverish the water and reduce one or more of the inorganic nutrients to a level favorable for the growth of dinoflagellates (but too low for the diatoms), and also allow the production of organic nutrients such as vitamin B12, which are important for their growth.
Dinoflagellates and oil and coal deposits
Blooms of dinoflagellates have most likely been responsible for some of the oil deposits of the world, including the North Sea oil deposit (Downie, 1956; Gallois, 1976). The best studied oil
deposits are the oil shales of the Kimmeridge Clay deposits in England, which vary from less than 100 m in thickness to over 500 m and are composed of a number of different layers: clays and black and brown shales intermixed with occasional thin limestones. The limestones are composed primarily of coccolithophorids, whereas the shales contain yellow-brown organic matter called kerogen. Kerogen contains a large amount of amorphous organic matter, from which it is impossible to determine its biological origin. In addition to the amorphous organic matter, there are found large numbers of dinoflagellates and their hystrichospores, as well as some coccoliths. The best known Kimmeridge Clay deposit is the Kimmeridge Coal, a highly bituminous shale, about 80 cm thick. The Kimmeridge Coal has long been used as a solid fuel, and in the latter half of the last century much interest was taken in it as a source of oil. However, the high sulfur content and the relative thinness of the “coal” bed prevented the economic exploitation of the oil.
The Kimmeridge Clay oil shales were formed from algal blooms in seas that were to some

DINOPHYTA 285
Fig. 7.37 The structure of dinosterol, the major sterol in
dinoflagellates.
degree land-locked, with salinity a little beneath the average salinity of the open ocean. These seas were rich in land-derived nutrients, allowing the water to support blooms of toxic dinoflagellates. These blooms deoxygenated and poisoned the water, providing the temporary anaerobic bottom conditions required for the preservation of organic matter. It is interesting that one of the bivalves commonly poisoned by toxic dinoflagellate blooms today is Lucinoma borealis, and the bivalve that forms the most extensive plasters in the Kimmeridge Clay oil shales is the related
Lucina miniscula.
Petroleum deposits and ancient sediments contain 4 -methylsteroidal hydrocarbons, which probably originated from 4 -methylsterols in dinoflagellates (Robinson et al., 1984). The dinoflagellates differ from other classes of algae with respect to the dominance of 4 -methylsterols among their sterol components and the uniqueness of certain of these 4 -methylsterol structures (Leblond and Chapman, 2002; Giner et al., 2003). The principal sterol of several marine dinoflagellates, and organisms with dinoflagellate symbionts, is dinosterol (Fig. 7.37).
Bioluminescence
About, about, in reel and rout The death-fires danced at night The water, like a witch’s oils Burnt green, and blue, and white
Samuel Taylor Coleridge
The Rime of the Ancient Mariner
Fig. 7.38 A possible partial structure of dinoflagellate
luciferin. (After Dunlap and Hastings, 1981; Hastings, 1986.)
Mariners from early times have marveled at the displays of bioluminescence that accompany large populations of dinoflagellates. The burning seas were at first thought to be of supernatural origin, omens of the pleasure or displeasure of the gods. As science began to usurp the explanation of natural phenomena from religion, the light emitted from friction between salt molecules or from phosphorus burning in water was invoked; the term phosphorescence still survives today from the explanation. By 1800, living cells were suspected, but the last experiments were not settled in favor of a biological origin until 1830 (Sweeney, 1979).
There are two types of light emission in living organisms: (1) bioluminescence (chemiluminescence), in which energy from an exergonic chemical reaction is transformed into light energy; and (2) photoluminescence, which is dependent on the prior absorption of light (Hastings, 1986). Many marine, but no freshwater dinoflagellates are capable of bioluminescence. The Dinophyceae are the main contributors to marine bioluminescence, emitting a bluish-green (maximum wavelength at 474 nm) flash of light of 0.1-second duration when the cells are stimulated. The luminescent wake of a moving ship or the phosphorescence of tropical bays is usually caused primarily by Dinophyceae.
The compound responsible for bioluminescence is luciferin (Fig. 7.38), which is oxidized with the aid of the enzyme luciferase, resulting in the emissions of light. Luciferin and luciferase are terms for a general class of compounds, and not of a specific chemical structure. Bioluminescence occurs in many organisms in many different phyla, ranging from bacteria to dinoflagellates to jellyfish and brittle stars to worms, fireflies, molluscs, and fish (Hastings, 1986). In bacteria, luciferin is a reduced flavin; in insects it is a (benzo)thiazole nucleus; and in dinoflagellates it is a tetrapyrrole.

286 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Likewise, luciferase has different structures, although all luciferases share the feature of being oxygenases (enzymes that add oxygen to compounds). The necessity for oxygen in bioluminescence was actually discovered by Boyle in 1667, who showed with his air (vacuum) pump that:
a piece of shining (bioluminescent) wood . . . gave a vivid light . . . which was manifestly lessened . . . [at] about the seventh suck, losing its light more and more as the air was still further pumped out. . . .
Wherefore we let in outward air and had the pleasure to see the seemingly extinguished light revive so fast and perfectly, that it looked to us almost like a little flash of lightening. (Cited in Harvey, 1952, p. 142).
In the basic reaction of bioluminescence (Hastings, 1983), a luciferin is oxidized by a luciferase, resulting in an electronically excited product (P)* which emits a photon (h ) on decomposition:
luciferase
luciferin O2 → (P)* → P h
Luciferin in the dinoflagellates has been reported to be a linear tetrapyrrole (Fig. 7.38). Associated with dinoflagellate luciferin is a luciferin-bind- ing protein (LBP) which sequesters luciferin at alkaline pH and releases it under acidic conditions (Sulzman et al., 1978). It has been postulated that the flash of bioluminescent light may occur simply by a lowering of the pH from 8.0 to 6.5. Agitation of cells depolarizes the vacuolar membrane, allowing a flux of protons (H ) and acidification of the peripheral cytoplasm (Johnson et al., 1985). Lowering the pH causes two pH-dependent
Fig. 7.39 The white spots in these photographs of Lingulodinium cells indicate fluorescence and thus localization of luciferin, the substrate that reacts with the enzyme luciferase and oxygen to produce light. The intensity of the luciferin fluorescence is greater in the night phase (left) of the circadian cycle of bioluminescence than in the day phase (right). The amount of luciferase shows a similar oscillation during the daily cycle. (From Johnson et al., 1985.)
reactions to occur: (1) release of luciferin from its binding protein at acidic pH, and (2) activation of luciferase followed by emission of a photon of blue-green light.
Luciferin, luciferase, and luciferase-binding protein occur in particles called scintillons (flashing units) that are approximately 0.5 to 1.5 m in diameter (Sweeney, 1980). Scintillations occur in cytoplasmic invaginations in the vacuolar membrane. Flashes of blue-green light are produced when an action potential proceeds along the vacuolar membrane, causing an outflow of protons from the acidic contents of the vacuole. The resulting drop in the pH in the scintillon causes flashes of light (Nicolas et al., 1991).
In Lingulodinium polyedrum (Figs. 7.39, 7.40), most bioluminescence occurs during the night phase of a circadian rhythm (Fig. 7.41) (Fritz et al., 1991). This is due to a ten fold increase in luciferase and luciferin-binding protein during the night phase. Daytime photoinhibition of bioluminescence is considered a mechanism to conserve the energy of cells when ambient light levels are high enough to render the bioluminescent flash ineffective (Buskey and Swift, 1983). The position of bioluminescence in Pyrocystis fusiformis (Fig. 7.40(a)) varies over 24 hours (Sweeney, 1980; Seo and Fritz, 2000). During the day, bioluminescence emanates from a spherical mass of tightly packed vesicles near the nucleus, whereas at night bioluminescence occurs in the peripheral cytoplasm. There is a reverse movement of chloroplasts, with the organelles in the cell periphery during the day and the chloroplasts concentrating around the nucleus at night.
DINOPHYTA 287
Fig. 7.40 (a) Pyrocystis fusiformis.
(b) Lingulodinium polyedrum (5
Gonyaulax polyedra).
(a) |
(b) |
Dinoflagellates can emit light in three modes:
(1) they can flash when stimulated mechanically, chemically, or electrically; (2) they can flash spontaneously; and (3) late at night they can glow dimly (Sweeney, 1979). The maximum amount of light emitted in a flash differs widely among species, with larger species emitting more light per flash than smaller ones. In a population of dinoflagellates, the cells emit an average of one flash per cell per day (Hastings and Krasnow, 1981). It is not clear whether each cell flashes once and only once during this period, or whether some cells are responsible for a larger share of the flashes whereas others do not emit at all. The nutritional status of a cell influences the brightness of the flash. Noctiluca with green algal symbionts (Fig. 7.54) shows an increase in the photons emitted per flash as the intensity of the illumination of the dinoflagellate, and therefore photosynthesis, increases. Different isolates of the same species in genera such as Dissodinium and Pyrocystis (Figs. 7.40(a), 7.49(c), (d)) can be bioluminescent or not (Swift et al., 1973; Schmidt et al., 1978).
Pyrocystis (Figs. 7.40(a), 7.49(c), (d)), one of the most strongly phosphorescent dinoflagellates, is the chief source of diffused phosphorescence in the sea in equatorial regions (Swift et al., 1973). Species of Pyrocystis produce at least 1000 times more bioluminescence per cell than members of the genus Lingulodinium (Figs. 7.39, 7.40) and about 100 times as much light per cell as Ceratium fusus, Peridinium pentagonium, and Pyrodinium bahamense.
There are two theories concerning the adaptive value of bioluminescence to dinoflagellates, both of which relate to nighttime grazing of the dinoflagellates:
1“Burglar alarm” hypothesis. This hypothesis argues that dinoflagellates render themselves
dangerous as prey to invertebrate grazers because they generate a signal identifying the location of invertebrate food to individuals two levels up the food chain from the dinoflagellates (Abrahams and Townsend, 1993). Bioluminescence generated by dinoflagellates serves to attract predators of the grazers of the dinoflagellates.
2“Startle” hypothesis. In this hypothesis, mechanical stimulation of a bioluminescent dinoflagellate by a grazer produces a flash of light that startles an invertebrate grazer, such as a copepod, and causes the copepod to swim away with its feeding appendage retracted (Buskey and Swift, 1983).
Whichever theory is correct, experiments have shown that copepods consume only half as many dinoflagellates at night, indicating that the bioluminescence is serving as a deterrent to grazing (Buskey et al., 1983).
Rhythms
Many Dinophyceae exhibit rhythmic processes, with the best known of the rhythms in the algae being those in the dinoflagellate Lingulodinium polyedrum (Figs. 7.39, 7.40) (Sweeney, 1969). It produces light via bioluminescence by forming its own dinoflagellate-specific luciferin and luciferase. The cells emit a flash of light when the seawater in which they are swimming is given a sharp shake or stirred vigorously. When the luminescence is measured while the culture is being stirred, the amount of light that the cells emit is not always the same and depends upon their recent history. If they have been growing in natural illumination or on a light–dark cycle, the amount of light emitted will be markedly

288 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.41 The rhythm of stimulated luminescence in
Lingulodinium polyedrum measured in (a) a light–dark cycle of 12:12 and (b) in complete dark. (After Sweeney, 1969.)
dependent on the time of day when measurements are made. If luminescence is elicited during the day, the amount of light will be low, and very hard stirring will be required to bring about any luminescence at all. But if the cells are stimulated at night, a great deal more light will result, and the most delicate shake will produce a flash. When the amount of luminescence is plotted as a function of the time of day, a graph such as that in Fig. 7.41(a), will result. The greatest luminescence will be produced in the middle of the dark period, whereas toward morning, flashes will gradually become smaller and a greater stimulus will be required. The rhythm is circadian (which means literally about (circa) a day (diem)), as shown by the persistence of changes in brightness of luminescence when the cells are kept in the dark (Fig. 7.41(b)). In continuous darkness, the cycle continues for as long as 4 days, but the
amplitude becomes successively smaller. If the cells are kept in continuous light, the reduction in amplitude is no longer evident. Cycles continue for at least 3 weeks in continuous light of appropriate intensity (Fig. 7.42).
When photosynthesis in Lingulodinium polyedrum is measured, either as oxygen production or carbon dioxide fixation, it is also found to be rhythmic (Fig. 7.45). The rhythm is circadian and continues under conditions of continuous light. The maximum rate of photosynthesis occurs, as one would expect, in the middle of the day. The rhythm in photosynthesis is due to changes in photosystem II (Samuelsson et al., 1983) (Fig. 7.43).
A third rhythm with a circadian period in
Lingulodinium polyedrum is that of cell division (Fig. 7.45); all cell division occurring during 30 minutes when cultures are in a light–dark cycle. When the light–dark cycle is 12 : 12, then this

DINOPHYTA 289
Fig. 7.42 The rhythm of stimulated luminescence in
Lingulodinium polyedrum measured in continuous light (1000 lux). (After Sweeney, 1969.)
Fig. 7.43 Beatrice M. Sweeney Dr. Sweeney’s investigations established the rhythmic phenomena of dinoflagellates. Born August 11, 1914, in Boston, Massachusetts. Died June 30, 1989 in Woods Hole, Massachusetts. She received her A.B. from Smith College (1936) and her Ph.D. from Radcliffe College (1942). She worked as a laboratory and research assistant at the Mayo Clinic (1942–43) and Scripps Institution of Oceanography (1947–55). From 1961 to 1967, she was a biologist and lecturer at Yale University. In 1967 she moved to the Department of Biological Sciences at the University of California, Santa Barbara. She published two editions of her book Rhythmic Phenomena in Plants. (Photo from Garbary and Wynne, 1996.)
30 minutes spans “dawn.” An investigation of other light–dark cycles, such as 7 : 7, shows that the dark–light transition is not the determining factor because division takes place about 12 hours after the beginning of the dark period, even though this time is considerably after the beginning of the next light period. In continuous light of low intensity where other rhythms of Lingulodinium polyedrum persist, very little cell division takes place, and the average generation time may be as long as 6 days. However, those cells that are ready to divide do so only at the expected time in each 24 hours.
A fourth type of rhythm involves the vertical migration of dinoflagellate cells in the water column (Eppley et al., 1968; Horiguchi and Pienaar, 1988; Lombard and Capon, 1971; Roenneberg and Deng, 1997). Before dawn, the cells rise to the surface, where they form dense clouds (aggregations), and before night fall, they again sink to lower depths (Fig. 7.44). In the marine environment, this vertical migration exposes the cells to several gradients: (1) Nutrients are more concentrated at lower depths (accumulating at the bottom of the ocean or at thermoclines) while surface waters are often practically devoid of nutrients. (2) Temperatures at the surface exceed those in deeper waters. (3) Variations in light intensities. (4) In shallow waters, differences in washout by the tidal waters.
In Lingulodinium polyedrum, there exists a control over luminescence, photosynthesis, and cell division, so that each process reaches a maximum and then declines in an orderly fashion during each 24 hours (Fig. 7.45). A single process, a biological clock, may control all of these processes. It appears that the part of the cell that may be the controlling agent is the plasma membrane

290 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.45 Circadian rhythmicity has been identified in at least four distinct biological processes of the unicellular
Lingulodinium polyedrum. Although the four rhythms peak at different times of the 24-hour day, as represented here diagrammatically, they are all synchronized with the circadian clock. Perturbations that reset the clock will phase-shift all four rhythms simultaneously, suggesting that all of these overt rhythms are driven by a single pacemaker. (After Johnson and Hastings, 1986.)
because there is a rhythmic reorganization of the plasma membrane over a 24-hour period in synchronized cells (Adamich et al., 1976).
Although the phases at which the rhythms peak in Lingulodinium polyedrum are different, it is generally believed that they are all controlled by a
Fig. 7.44 Profiles of chlorophyll a during a bloom of dinoflagellates showing the vertical migration of dinoflagellates at different times of the day. (After Eppley et al., 1968.)
single pacemaker (Johnson and Hastings, 1986). The rhythmic processes seem to have no feedback mechanism and thus appear to be driven systems. For example, photosynthesis can be completely inhibited with a specific herbicide, but the bioluminescence rhythm continues and its phase is not altered. Such observations on Lingulodinium polyedrum suggest models of circadian organization like those in Fig. 7.46. The central pacemaker is phased to the solar light cycle by means of a photoreceptor and an entraining pathway, and it controls the expression of the different rhythms, such as cell division, bioluminescence, and photosynthesis.
Two different systems time the circadian rhythm in dinoflagellates, a red-light sensitive
