
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index

DINOPHYTA 277
Fig. 7.27 (a) Diagram of a longitudinal section and cross sections of a charged trichocyst of
Lingulodinium polyedrum. The single membrane limiting the trichocyst is lined on its inner surface with fine hoops or spirals. Within the membrane is a crystalline core (c) composed of long rods or plates. Along the upper one-third of the core, short tubules (t) protrude downward and outward. At the anterior portion of the core, a series of fibers (f) attach to the core to still finer fibrils, which eventually reach to the anterior portion of the enclosing membrane. (b) Drawing of a segment of a wall plate of
Lingulodinium polyedrum. Pore-like thin areas in the plate contain two or three slightly ridged discs (d) through which the trichocyst is discharged. (c) Cell of Oxyrrhis with discharged trichocysts. (d) Drawing of the tip of a discharged trichocyst showing striations. ((a),(b) after Bouck and Sweeney, 1966.)
Accumulation body
This is a large vesicle containing the remains of digested organelles (Figs. 7.36(c), 7.53(b)). It is probably similar to the Corps de Maupas of the Cryptophyceae and the digestive vesicles of other flagellates (Zhou and Fritz, 1994). An accumulation body is particularly common in symbiotic Dinophyceae.
Resting spores or cysts or hypnospores and fossil Dinophyceae
The resting spore or cyst of most dinoflagellates is morphologically distinct from the parent cell. They are 30 to 70 m in diameter with smooth or spinose bodies (Figs. 7.28, 7.36). The
newly formed cysts of Scrippsiella trochoidea have ten times more carbohydrate and 1.5% the respiratory rate of vegetative cells (Brooks and Andersen, 1990).
The cell walls of the cysts are highly resistant to decay and contain dinosporin, a chemical similar to sporopollenin in the pollen of higher plants (Kokinos et al., 1998). The cysts are extremely resistant and are preserved in ancient sediments where they are of value in paleoecological studies. Fossilized cysts are called hystrichosphaerids (hystrichospores) and are included in fossilized cyst-like structures of unicellular algae called acritrachs. These fossilized dinoflagellates first appeared in the Triassic and reached a peak in the Jurassic and Cretaceous, followed by a decrease in the Tertiary (MacRae et al., 1996).

278 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.28 Dinoflagellate cysts. (a) Alexandrium catenella. (b)
Calciodinellum operosum. (c) Scrippsiella trophoidea. (d)
Gonyaulax grindleyi. (e) Polykrikos schwartzii. (f) Gymnodinium catenatum. ((d)–(f) from Ellegaard et al., 1994; (b) from Montresor et al., 1997; (a),(c) from Meksumpun et al., 1994.)
Hystrichosphaerids were discovered independently by paleontologists and classified under a separate taxonomic scheme consisting of only fossil species. Many extant resting spores are identical to hystrichosphaerids of the Tertiary and Quarternary, with the result that there are two names for the same structure.
The process of encystment or resting spore formation is regulated by a complex interaction of day length, temperature, and nutrient concentration (Kremp, 2001; Sgrosso et al., 2001). Melatonin levels increase by several orders of magnitude during encystment and may function in preventing oxidation of the lipids in the cyst (Balzer and Hardeland, 1996). In the freshwater dinoflagellate Woloszynskia tylota, encystment involves the following changes (Bibby and Dodge, 1972): (1) replacement of the theca by a thin
amorphous outer wall, which gradually thickens by the deposition of material on its inner face; (2) the appearance of a layer of closely packed lipid droplets at the cytoplasmic margin of the mature cyst; (3) the reduction in size or disappearance of cytoplasmic structures such as chloroplasts, Golgi bodies, and pusules; and (4) the enlargement of a central orange-brown body and cytoplasmic vacuoles containing crystals. Cysts are recognizable because they lack chromatophores and have a microgranular brown cytoplasm and a red eyespot (if the organism normally has an eyespot). Calcification of cysts in some genera occurs by the deposition of calcium carbonate crystals in the narrow space between the cell wall and the plasma membrane (Montresor et al., 1997). The cysts of Ceratium hirundinella contain an outer silicon layer (Chapman et al., 1982).
A small number of dinoflagellates produce siliceous skeletons, with the protoplasm wrapped around the skeleton. The best known species is the heterotrophic non-armored Actiniscus pentasterias (Fig. 7.29) (Hansen, 1993).

DINOPHYTA 279
Fig. 7.29 Actiniscus pentasterias. (a) Scanning electron micrograph of the siliceous internal skeleton (two pentasters). (b) Transmission electron micrograph showing two pentasters surrounding the nucleus. (From Hansen, 1993; Preisig, 1994.)
Toxins
Some Dinophyceae have the ability to produce very potent toxins which cause the death of fish and shellfish during red tides when there are dinoflagellate blooms that color the water red. The dinoflagellates become lodged in the gills of the shellfish, and when shellfish are eaten by humans or animals, poisoning results.
Historically, red tides and paralytic shellfish poisoning have been mentioned many times (Shilo, 1967). One of the plagues that struck Egypt was described in the Bible: “all the waters that were in the river were turned to blood. And the fish that was in the river died; and the river stank, and the Egyptians could not drink the water of the river . . .” (Exodus 7 : 17). This description is strongly reminiscent of the poisonous red tides. Darwin, in his description of discolored water in 1832 during his voyage on the Beagle, graphically described blooms of algae that were dinoflagellates.
Death and illness caused by consumption of poisonous mussels and clams were reported by Captain Cook and Captain George Vancouver
during their expeditions to the coast of the Pacific Northwest. An old custom among Indian tribes along the coast of Alaska was to station sentries to watch for the marine luminescence occurring during hot weather, which they understood to be associated with Kal-Ko-O, their name for mussel poisoning.
All of the dinoflagellates that have been convincingly demonstrated to produce toxins contain chloroplasts, indicating that the ability to produce toxins may have been derived from endosymbiotic cyanobacteria.
1Diarrhetic shellfish poisoning. This occurs primarily in temperate regions and is caused by species of the planktonic dinoflagellates
Exuviaella, Dinophysis (Fig. 7.30(a), (b)), and
Prorocentrum (Figs. 7.30(c), 7.55, 7.56(c)). Diarrhetic shellfish poisoning is caused by the polyether carboxylic acids okadaic acid, macrolide toxins, and yessotoxin (Figs. 7.31, 23.3, 23.4). The toxins are powerful inhibitors of serineand threonine-protein phosphatases PP1 and PP2A and induce severe gastroenteritis (Zhou and Fritz, 1994; Morton and Tindall, 1995; Suzuki et al., 1997).
The polyether carboxylic acid okadaic acid is initially formed inside the dinoflagellate cell as dinophysistoxin-4 (Fig. 7.31). This weakly sulfated derivative of okadaic acid is not toxic to the dinoflagellate cell. Dinophysistoxin-4 is

280 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.30 Scanning electron micrographs of dinoflagellates that cause diarrhetic shellfish poisoning. (a) Dinophysis acuminata. (b) Dinophysis fortii. (c) Prorocentrum lima with
the arrows pointing to pores in the theca. (From Hallegraeff, 1993.)
Fig. 7.31 Dinophysistoxin-4 is produced in the dinoflagellate cell and released to the environment, where it is hydrolyzed to okadaic acid diol ester. Okadaic acid diol ester is lipid soluble and passes through the cell membrane of the shellfish where it is hydrolyzed to toxic okadaic acid.

DINOPHYTA 281
Fig. 7.32 Scanning electron micrographs of Gambierdiscus toxicus, the causative organism of ciguatera fish poisoning. (a) Epithecal view. (b) Hypothecal view. The numbers refer to arrangements of the plates. (From Faust, 1995.)
either excreted by the dinoflagellate cell, or is released on death of the cell. Dinophysistoxin- 4 is hydrolyzed in the medium to okadaic acid diol ester, which is lipid soluble and can pass through cell membranes. Thus, okadaic acid diol ester can be taken up by cells which further hydrolyze the compound to okadaic acid. Research has shown that okadaic acid with a free acid moiety is the toxic form of the compound (Hu et al., 1999; Windust
et al., 2000).
2Ciguatera fish poisoning. This occurs primarily in tropical regions with the common causative agent being Gambierdiscus (Figs. 7.6, 7.32) which is common circumtropically between 32° N and 32° S. The dinoflagellate contains gambieric acids, ciguatoxins, and maitotoxins (Fig. 7.33), putative Ca2 channel activators that result in breakdown of the cell membrane (Igarashi et al., 1999). Gambierdiscus is epyphytic on macroalgae that are eaten by herbivorous fish and shellfish, which, in turn, are eaten by humans. In French Polynesia alone, approximately 1000 cases of ciguatera fish poisoning are reported every year (Chinain et al., 1997). The term ciguatera is derived from the Spanish term “cigua” for the turban shell, which was commonly eaten before the illness developed. The typical course of ciguatera fish
poisoning is diarrhea for two days, followed by general weakness for one to two days. Occasionally the condition is fatal (Withers, 1982).
3Paralytic shellfish poisoning. This is caused by species of Alexandrium (A. catanella, A. acatenella, A. excavatum (Fig. 7.35), A. tamarensis), Pyrodinium bahamense (Fig. 7.34), and Gymnodinium catenatum (Fig. 7.34). These dinoflagellates produce a group of toxins that are derivatives of saxitoxin (Fig. 23.2). Saxitoxins are potent neurotoxins acting upon voltage-gated Na -channels, preventing influx of Na , thereby preventing the generation of an action potential (Cembella, 2003).
Alexandrium excavatum is a toxic red-tide dinoflagellate (Fig. 7.35). The vegetative cells divide to produce motile gametes that subsequently fuse. In the early stages of gamete fusion, when superficial contact is apparent, fusing pairs swim poorly and settle in the water. The motile quadriflagellate planozygotes swim for a few days before losing their flagella and thecal plates, and encyst to form resting cysts (hypnospores) (Figs. 7.28(a), 7.36) that can survive for 5 to 15 years. The hypnospores contain storage products and have a thick, three-layer wall (Kennaway and Lewis, 2004). Cyst germination is controlled by a biological clock with a 12 month maturation period (Perez, et al., 1998). Each hypnospore undergoes meiosis with two cell divisions to produce haploid vegetative cells, completing the life cycle (Destombe and Cembella, 1990; Nagai et al., 2003).
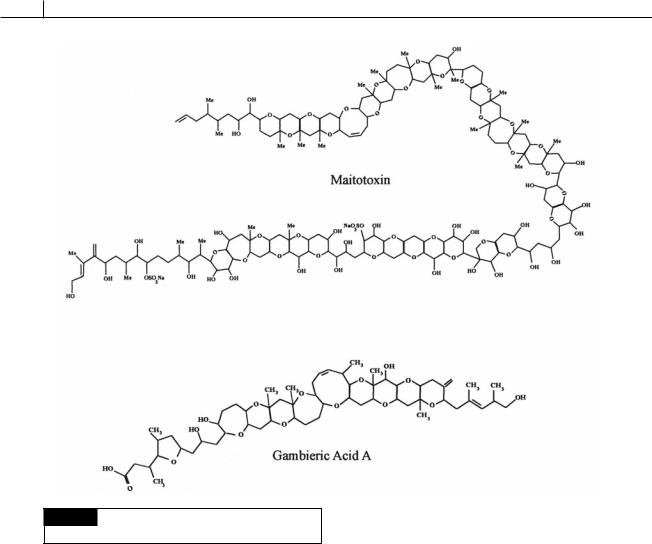
282 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
Fig. 7.33 The chemical structure of maitotoxin and
gambieric acid.
The amount of toxin in cells of Alexandrium is relatively low when nutrients are in ready supply (John and Flynn, 2002). The toxin concentration is highest under conditions of phosphorus deficiency, possibly because free amino acids (precursors of toxins) accumulate under conditions of phosphorus deficiency.
Cells of dinoflagellates that produce toxins are avoided by grazing copepods, indicating that the extra energy put into toxin production is more than offset by the decreased grazing of the dinoflagellate cells (Guisande et al., 2002).
A number of factors have been suggested as the cause of red tides:
1High surface-water temperatures:
Dinoflagellates favor warm water, and are
generally more abundant near the surface. This does not necessarily mean that they occur only in warm seas, because the surface of the sea in normally cool areas may be warmed up during periods of hot, calm weather.
2Wind: A strong, offshore wind aids upwelling, whereas a gentle onshore wind concentrates the bloom near the coast. On the other hand, heavy weather and strong winds disperse the bloom. Storms also result in the death of dinoflagellates and can prevent the development of red tides (Juhl and Latz, 2002;
Sullivan and Swift, 2003).
3 Light intensity: There is usually a period of bright, sunny, calm weather before outbreaks.
4Nutrients: Red tides usually occur after an upwelling has stopped, but the nutrients brought to the surface do not, themselves, appear to be the direct cause of these blooms (Grindley and Nel, 1970). It is thought that

DINOPHYTA 283
Fig. 7.34 Scanning electron micrographs of poisonous dinoflagellates. Alexandrium minutum
(upper left), Karlodinium veneficum
( Gymnodinium galatheanum) (upper right), Gymnodinium catenatus
(bottom left), Pyrodinium bahamense var. compressum (bottom right).
Karlodinium micrum has killed cagereared fish, while the others cause paralytic shellfish poisoning.
(From Hallegraeff, 1993.)
Fig. 7.35 The life cycle of
Alexandrium excavatum. (Adapted from Destombe and Cembella, 1990.)
