
The Physics of Coronory Blood Flow - M. Zamir
.pdf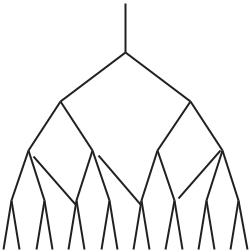
1.6 Branching Structure |
17 |
Fig. 1.6.3. When interconnections occur sporadically (dashed lines) in an otherwise open tree structure, they are referred to as “collateral” vasculature. The presence of collateral vasculature has been demonstrated in the coronary circulation of the human heart (see Figs. 1.6.4–6) but controversy continues over its origin, rate of growth, and hemodynamic significance. Collateral vasculature is to be distinguished from permanent collateral bridges such as the communicating arteries in the circle of Willis of the human brain. The latter are part of the fluid dynamic design of the cerebral circulation, that is, part of the normal anatomy of the cerebral vasculature. Collateral vasculature in the human heart is not part of the normal anatomy of the coronary vasculature.
The overwhelming majority of heart failures caused by insu cient myocardial blood supply [185, 173, 27, 15, 69, 16, 74, 95] point clearly to a failure of this mechanism in these cases. The di erence between the time scale of development and growth of collateral vessels and the time scale of vascular obstruction, whether by a slow disease process or sudden occlusion, is clearly an important factor. The indications are that the mechanism of collateral vasculature cannot deal with sudden occlusion, first because the vasculature would likely not be present before the occlusion occurs, and second because new vasculature cannot develop within the fast time scale of a sudden occlusion. In the case of the much slower pace of stenosis by atherosclerotic disease, the indications are that collateral vasculature can develop in time to make a significant contribution, but whether it does or not in every case, and the magnitude of the contribution it makes in each case, is not clear. The subject is decidedly far from settled [172, 44, 180, 77].
The ultimate question, of course, is the evolutionary origin of the mechanism of collateral vasculature in the human heart. Is it an integral part of the hemodynamic design of the coronary circulation, or merely a normal angiogenic response to ischemia, not peculiar to the heart? If it is the former,
18 1 Static Design Issues
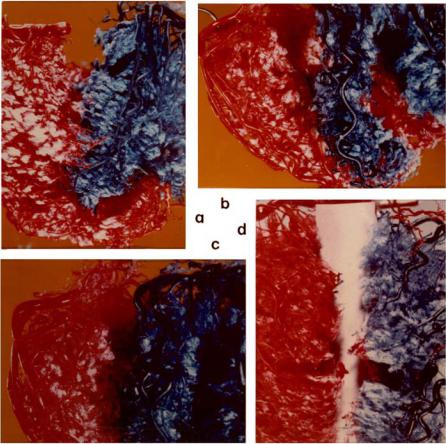
Fig. 1.6.4. Resin casts showing sporadic collateral vasculature between branches of the left and right coronary arteries in four human hearts. The two sides were perfused in di erent colours to uncover places where mixing of the two colours occured. This mixing could not be mediated by capillary beds since the size of dye particles prevented them from entering these vessels. Thus, the capillary beds actually acted as a barrier between the two colours because only clear resin could enter these beds (seen as white flu in (a,b)). This ensured that any observed mixing occured at higher levels of the tree structure. From [216]. (See color insert.)
1.6 Branching Structure |
19 |
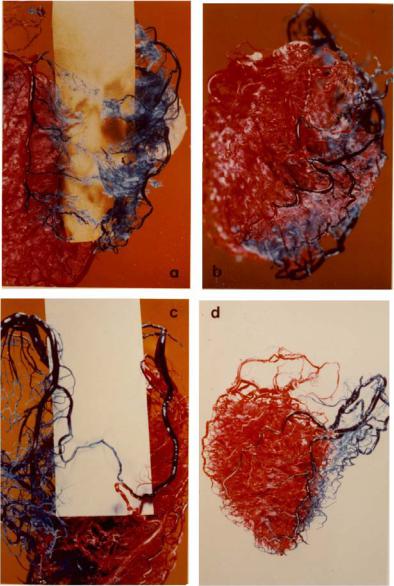
Fig. 1.6.5. Resin casts of four human hearts in which the left main and the right coronary arteries were perfused in di erent colours. Here it is seen that in the absence of collateral vasculature, the distributions of the left and right coronary arteries are demarcated by clear borders, and no mixing of the two colours is observed. See also caption for Fig. 1.6.4. From [216]. (See color insert.)
20 1 Static Design Issues
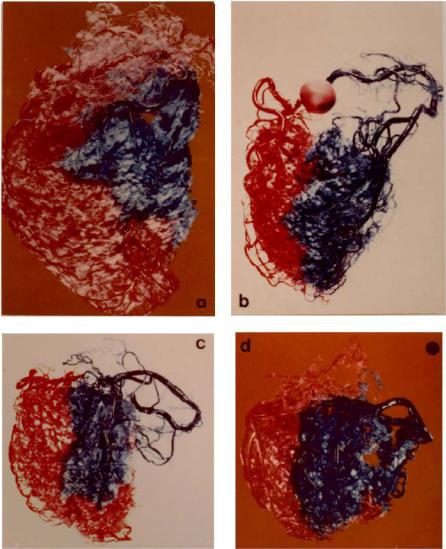
Fig. 1.6.6. The absence of collateral connections is further confirmed by the ability to physically separate the distributions of the left and right coronary arteries. In fact, in (b) and (d) it is seen that the beds of individual vessels on the same side could also be separated from each other. See also caption for Fig. 1.6.4. From [216]. (See color insert.)
collateral vessels would likely have a more permanent, less sporadic, presence within the coronary vasculature. Yet, such permanent collateral bridges as the communicating arteries in the circle of Willis of the brain do not exist in the heart. Studies of the branching architecture of the coronary arteries show repeatedly that the underlying branching pattern is that of an open tree structure [227, 223, 214, 106]. Sporadic connections between branches can be easily demonstrated, particularly in the presence of ischemic disease or history (Fig. 1.6.4). Under normal circumstances, however, the indications are that the distributions of the main coronary arteries are fairly distinct and do not mesh with each other (Figs. 1.6.5, 6). Meshing does occur at the cap-
1.7 Underlying Design? |
21 |
illary level, of course, but at that level it can no longer serve the function of collateral vasculature since the meshing is highly localized and cannot reach (hemodynamically) from the distribution territory of one major artery to that of the next.
1.7 Underlying Design?
Wide variability in the anatomical layout of the coronary arteries and their major branches calls into question the “naming” of these vessels, because the names imply that they are clearly defined anatomical entities. Yet we have seen that the left or the right coronary artery in one heart is not the same functional entity as the left or the right coronary artery in another. This variability also calls into question the notion of “one-artery” or “twoartery” disease as measures of the severity of coronary heart disease, since, again, these terms suggest that one artery in one heart is the same functional entity as an artery with the same name in another heart, or indeed that two arteries have twice the functional value of one artery. More accurately, wide variability in their size and distribution suggests that the coronary arteries, as represented by their anatomical names, do not represent elements of the underlying functional design of the coronary network as a fluid conveying system. Elements of that functional design would be represented by features of the coronary network which do not vary from heart to heart.
The situation is not unlike the functional design of the heart itself as a double pump for the maintenance of two circulations. The characteristic four chambers of the heart are essential elements of this design that do not change from heart to heart. On the other hand, the exact size and shape of these chambers, or the size and shape of the heart as a whole, are secondary features that vary considerably from heart to heart. They are not essential elements of the underlying design of the heart as a double pump. Thus, by analogy, we ask: are there features of the coronary network that do not change from heart to heart?
A study of human hearts with the purpose of addressing this issue found, in summary, that the coronary network serves the heart by dividing the myocardium into six distinct zones [228]. Each zone is served by two types of vessels: “distributing vessels” that run along the borders of these zones, and “delivering vessels” that enter the zones and branch profusely to reach the capillary bed and thereby deliver blood within the zone. Indeed, subsequent studies confirmed that there is a significant di erence in the branching patterns of these two types of vessels, consistent with their di erent roles [214, 218]. Briefly, distributing vessels branch only sporadically and maintain their identity as they circle the zones, while delivering vessels quickly lose their identity as they branch profusely and more uniformly to reach the capillary beds (Figs. 1.7.1–3).

22 1 Static Design Issues
Fig. 1.7.1. A cast of the left anterior descending artery of a human heart and its branches. The artery runs along the anterior interventricular sulcus, as a distributing vessel, while its branches probe into the plane of the interventricular wall, as delivering vessels. This figure illustrates the di erence between the branching patterns of the two types of vessels. Distributing vessels branch only sporadically and maintain their identity, while delivering vessels quickly lose their identity as they branch profusely and more uniformly to reach the capillary beds. From [214].
1.7 Underlying Design? |
23 |
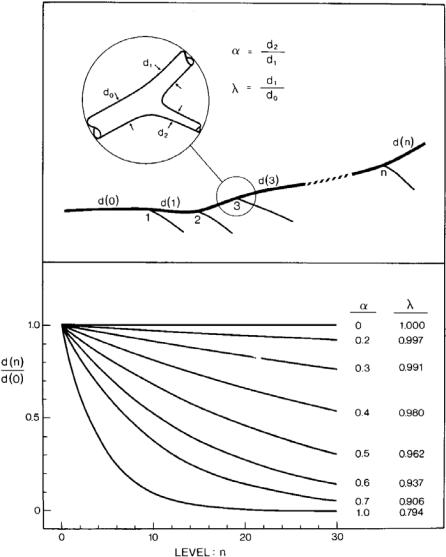
Fig. 1.7.2. A theoretical basis for the di erence between the branching patterns of distributing and delivering vessels. According to the cube law of branching, the diameter of an artery that undergoes repeated symmetrical or near symmetrical bifurcations diminishes rapidly, following one of the lower curves for which the value of the symmetry ratio α is near unity. The curves describe the diameter of the vessel at di erent levels n of a tree structure, or after undergoing n bifurcations. By contrast, the diameter of an artery that undergoes asymmetrical bifurcations, giving rise to relatively small side branches, diminishes much more slowly, following one of the top curves for which the value of α is near zero. From [214].
24 1 Static Design Issues
Borders defining the six zones of the myocardium in this scheme coincide with certain anatomical landmarks of the heart, namely the “sulci” of the two main dividing walls of the heart: the atrioventricular septum and the interventricular septum. Topographically, the sulci represent the intersections of the two dividing planes with the outer surface of the heart. They appear as faint grooves on the surface of the heart. The distributing vessels run along these grooves, usually covered by a thin layer of fat. The atrioventricular septum, for example, which lies in the coronary plane (Figs. 1.4.1, 2), produces the atrioventricular sulcus on the surface of the heart as a mark of its intersection with it. This sulcus runs as a “belt” around the “waist” of the heart and it is the groove along which the right coronary artery and the left circumflex artery run (Figs. 1.4.1, 2). The analogy used more commonly is that of a “crown” (presumably around the “head” of the heart), hence the term “coronary” as mentioned earlier.
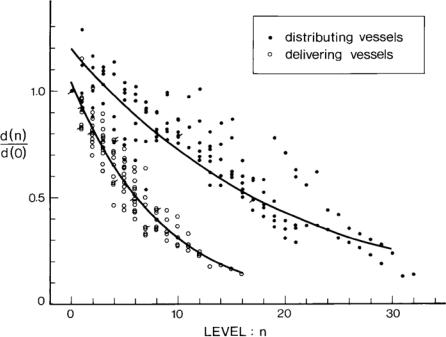
Fig. 1.7.3. Diameter measurements from casts of human coronary arteries. Measurements from distributing vessels are identified by bold circles while those from delivering vessels are identified by empty circles (see Fig. 1.7.2 for notation). The curves represent a statistical fit of the data points. The figure shows clearly that delivering vessels branch more profusely and undergo fewer bifurcations but their diameters diminish rapidly in the process. The diameters of distributing vessels, by contrast, diminish more slowly and the vessels undergo a larger number of bifurcations. From [214].
1.7 Underlying Design? |
25 |
Another important inner wall of the heart is the interventricular septum, which acts as a divider between the two ventricles but is functionally part of the left ventricle. The intersection of the interventricular plane with the outer surface of the heart produces the interventricular sulcus along which the anterior and posterior descending arteries run (Figs. 1.4.1, 5). By what appears to be a clear design, and in a pattern which does not vary from heart to heart, the two arteries circle the wall as distributing vessels, while branches from them run into the plane of the wall as delivering vessels (Fig. 1.7.1).
Two other borders coincide with the “acute margin” on the right side of the heart, and the “obtuse margin” on the left (Fig. 1.4.5). The names of the six zones and of the borders defining them are shown in Fig. 1.7.4.
The zones and borders are anatomical landmarks which are permanent features that do not change from heart to heart (Fig. 1.7.3). The underlying fluid dynamic design of the coronary network appears to be based on these landmarks. By this functional design, distributing arteries run along the dividing borders, giving rise to delivering branches that enter the zones and implement blood delivery. This scheme by which the coronary network serves the heart does not change from heart to heart [228].
Variability in the size and shape of the zones, and wide variability in the length and size of coronary arteries referred to earlier, do not alter this scheme. A border may be occupied by di erent distributing vessels in di erent hearts, but the scheme remains constant. The posterior interventricular sulcus, for example, may be occupied by a posterior descending artery arising from the right coronary artery or one arising from the left circumflex. The left posterior atrioventricular sulcus may be occupied fully by the left circumflex artery, fully by the right coronary artery, or partly by both. In these di erent scenarios there is wide variability in the length and size of the coronary arteries involved, but the underlying design of the coronary network remains the same. In all cases distributing vessels bring blood supply to the borders of zones while delivering vessels enter the zones and implement delivery. Only the identities of the vessels vary from heart to heart, hence these identities, as represented by the anatomical names of the vessels, do not represent accurate functional elements of the coronary network.
This subject is clearly important in coronary heart disease where one or more arteries may be a ected by disease that limits its fluid dynamic function. The foregoing discussion suggests that an accurate clinical assessment of coronary heart disease in a particular heart should be based not on the anatomical names of the a ected vessels but on the fluid dynamic role of each a ected vessel in the scheme of blood supply to that heart. What is clearly important is whether the a ected vessel is a distributing or a delivering vessel, and what particular position it occupies in that particular heart. Only then is it possible to deduce the e ect of the diseased vessels on particular zones of the heart, and ultimately on its ability to perform the heart’s pumping function.
26 1 Static Design Issues
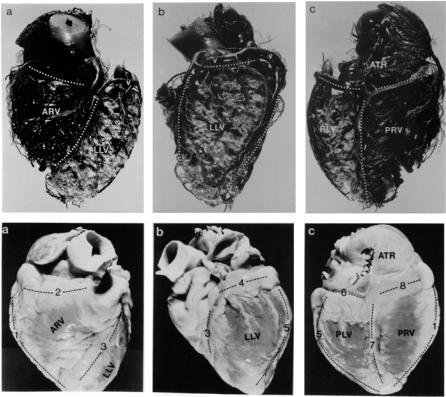
Fig. 1.7.4. Bottom row: a human heart in (a) an upright front view, (b) a left side view, and (c) a back view. Top row: cast of the coronary arteries of the same heart in the same views. Six zones of the myocardium are clearly visible in both sets of panels, defined by the distributing vessels seen in the top panels and by the bands of fat covering the distributing vessels in the lower panels. The borders in both cases are highlighted by dotted lines. A clear demonstration of how delivering vessels enter a zone and lose their identity is seen in panel (b) in the bottom row. Clear demonstrations of the way distributing vessels run along the borders of and thereby define the zones are seen in the top panels. The six zones are: anterior right ventricular (ARV), lateral left ventricular (LLV), posterior left ventricular (PLV), posterior right ventricular (PRV), atrial (ATR), and interventricular septal (IVS), not seen because it is an internal wall. From [228]
