Fatty Acids in Food: Saturated Versus Unsaturated
Fats consumed in the modern human diet vary widely in their fatty acid compositions. The table below provides a brief summary. The incidence of cardiovascular disease is correlated with diets high in saturated fatty acids. By contrast, a diet that is relatively higher in unsaturated fatty acids (especially polyunsaturated fatty acids) may reduce the risk of heart attacks and strokes. Corn oil, abundant in the United States and high in (polyunsaturated) linoleic acid, is an attractive dietary choice. Margarine made from corn, safflower, or sunflower oils is much lower in saturated fatty acids than is butter, which is made from milk fat. However, margarine may present its own health risks. Its fatty acids contain trans-double bonds (introduced by the hydrogenation process), which may also contribute to cardiovascular disease. (Margarine was invented by a French chemist, H. Mège Mouriès, who won a prize from Napoleon III in 1869 for developing a substitute for butter.)
Although vegetable oils usually contain a higher proportion of unsaturated fatty acids than do animal oils and fats, several plant oils are actually high in saturated fats. Palm oil is low in polyunsaturated fatty acids and particularly high in (saturated) palmitic acid (whence the name palmitic). Coconut oil is particularly high in lauric and myristic acids (both saturated) and contains very few unsaturated fatty acids.
Some of the fatty acids found in the diets of developed nations (often 1 to 10 g of daily fatty acid intake) are trans fatty acids— fatty acids with one or more double bonds in the trans configuration. Some of these derive from dairy fat and ruminant meats, but the bulk are provided by partially hydrogenated vegetable or fish
oils. Substantial evidence now exists to indicate that trans fatty acids may have deleterious health consequences. Numerous studies have shown that trans fatty acids raise plasma LDL cholesterol levels when exchanged for cis-unsaturated fatty acids in the diet and may also lower HDL cholesterol levels and raise triglyceride levels. The effects of trans fatty acids on LDL, HDL, and cholesterol levels are similar to those of saturated fatty acids, and diets aimed at reducing the risk of coronary heart disease should be low in both trans and saturated fatty acids.
H |
H |
|
C |
C |
|
|
|
O |
Oleic acid |
C |
cis double bond |
OH |
H
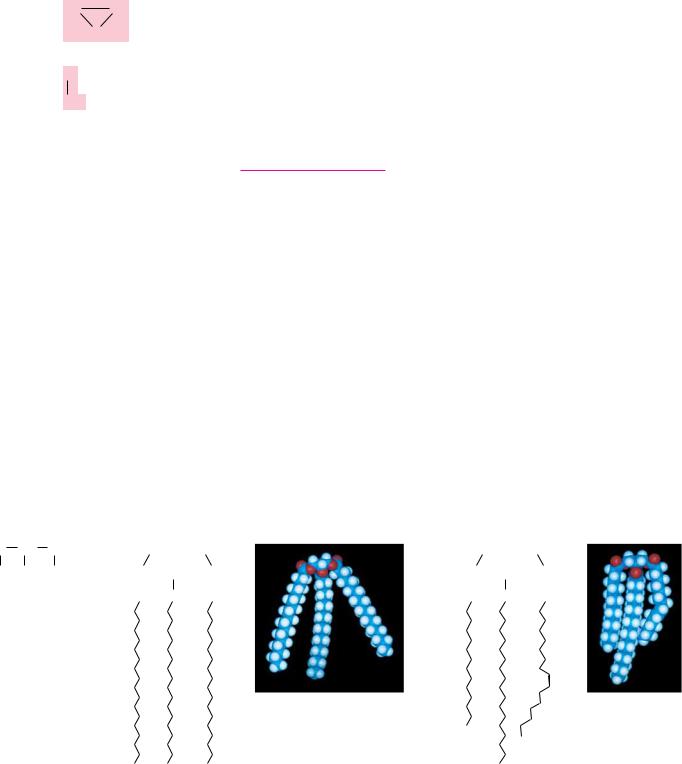
Elaidic acid trans double bond
Structure of cis and trans monounsaturated C18 fatty acids.
Fatty Acid Compositions of Some Dietary Lipids*
Source |
Lauric and Myristic |
Palmitic |
Stearic |
Oleic |
Linoleic |
|
|
|
|
|
|
Beef |
5 |
24–32 |
20–25 |
37–43 |
2–3 |
Milk |
|
25 |
12 |
33 |
3 |
Coconut |
74 |
10 |
2 |
7 |
— |
Corn |
|
8–12 |
3–4 |
19–49 |
34–62 |
Olive |
|
9 |
2 |
84 |
4 |
Palm |
|
39 |
4 |
40 |
8 |
Safflower |
|
6 |
3 |
13 |
78 |
Soybean |
|
9 |
6 |
20 |
52 |
Sunflower |
|
6 |
1 |
21 |
66 |
Data from Merck Index, 10th ed. Rahway, NJ: Merck and Co.; and Wilson, et al., 1967, Principles of Nutrition, 2nd ed. New York: Wiley.
*Values are percentages of total fatty acids.
8.3 ● Glycerophospholipids |
243 |
A D E E P E R L O O K
Polar Bears Use Triacylglycerols to Survive Long Periods of Fasting
The polar bear is magnificently adapted to thrive in its harsh Arctic environment. Research by Malcolm Ramsey (at the University of Saskatchewan in Canada) and others has shown that polar bears eat only during a few weeks out of the year and then fast for periods of 8 months or more, consuming no food or water during that time. Eating mainly in the winter, the adult polar bear feeds almost exclusively on seal blubber (largely composed of triacylglycerols), thus building up its own triacylglycerol reserves. Through the Arctic summer, the polar bear maintains normal physical activity, roaming over long distances, but relies entirely on its body fat for sustenance, burning as much as 1 to 1.5 kg of fat per day. It neither urinates nor defecates for extended periods. All the water needed to sustain life is provided from the metabolism of triacylglycerides (because oxidation of fatty acids yields carbon dioxide and water).
Ironically, the word Arctic comes from the ancient Greeks, who understood that the northernmost part of the earth lay under the stars of the constellation Ursa Major, the Great Bear. Although unaware of the polar bear, they called this region Arktikós, which
means “the country of the great bear.”
(Thomas D. Mangelsen/Images of Nature)
ond carbon is asymmetric. The various acylglycerols are normally soluble in benzene, chloroform, ether, and hot ethanol. Although triacylglycerols are insoluble in water, monoand diacylglycerols readily form organized structures in water (discussed later), owing to the polarity of their free hydroxyl groups.
Triacylglycerols are rich in highly reduced carbons and thus yield large amounts of energy in the oxidative reactions of metabolism. Complete oxidation of 1 g of triacylglycerols yields about 38 kJ of energy, whereas proteins and carbohydrates yield only about 17 kJ/g. Also, their hydrophobic nature allows them to aggregate in highly anhydrous forms, whereas polysaccharides and proteins are highly hydrated. For these reasons, triacylglycerols are the molecules of choice for energy storage in animals. Body fat (mainly triacylglycerols) also provides good insulation. Whales and Arctic mammals rely on body fat for both insulation and energy reserves.
8.3 ● Glycerophospholipids
A 1,2-diacylglycerol that has a phosphate group esterified at carbon atom 3 of the glycerol backbone is a glycerophospholipid, also known as a phosphoglyceride or a glycerol phosphatide (Figure 8.4). These lipids form one of the largest classes of natural lipids and one of the most important. They are essential components of cell membranes and are found in small concentrations in other parts of the cell. It should be noted that all glycerophospholipids are members of the broader class of lipids known as phospholipids.
The numbering and nomenclature of glycerophospholipids present a dilemma in that the number 2 carbon of the glycerol backbone of a phos-
244 Chapter 8 ● Lipids
O |
|
|
|
|
|
|
|
|
|
|
|
C |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
O C |
H |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
CH2 |
|
|
O |
|
P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
FIGURE 8.4 ● Phosphatidic acid, the parent compound for glycerophospholipids.
pholipid is asymmetric. It is possible to name these molecules either as D- or L-isomers. Thus, glycerol phosphate itself can be referred to either as D-glyc- erol-1-phosphate or as L-glycerol-3-phosphate (Figure 8.5). Instead of naming the glycerol phosphatides in this way, biochemists have adopted the stereospecific numbering or sn- system. In this system, the pro-S position of a prochiral atom is denoted as the 1-position, the prochiral atom as the 2-position, and so on. When this scheme is used, the prefix sn- precedes the molecule name (glycerol phosphate in this case) and distinguishes this nomenclature from other approaches. In this way, the glycerol phosphate in natural phosphoglycerides is named sn-glycerol-3-phosphate.
The Most Common Phospholipids
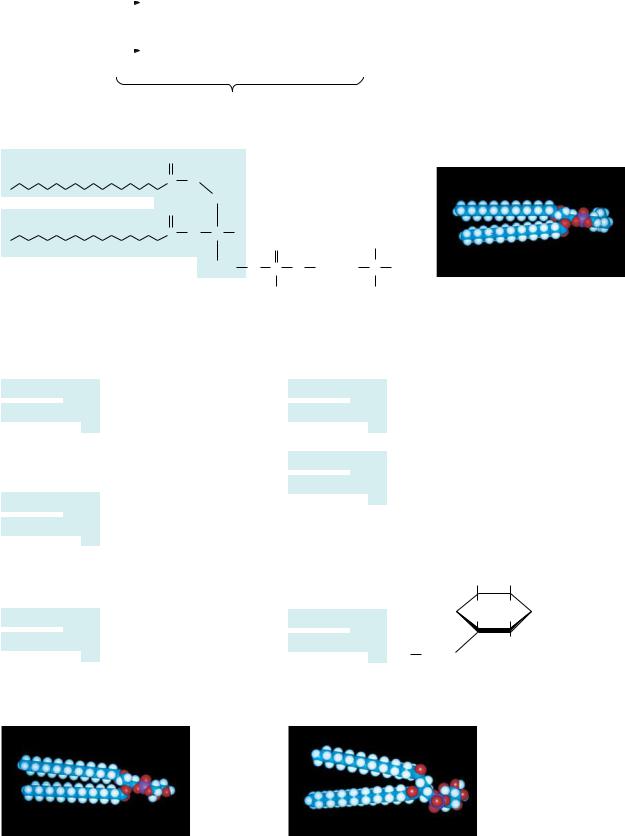
Phosphatidic acid, the parent compound for the glycerol-based phospholipids (Figure 8.4), consists of sn-glycerol-3-phosphate, with fatty acids esterified at the 1- and 2-positions. Phosphatidic acid is found in small amounts in most natural systems and is an important intermediate in the biosynthesis of the more common glycerophospholipids (Figure 8.6). In these compounds, a
A D E E P E R L O O K
Prochirality
If a tetrahedral center in a molecule has two identical substituents, it is referred to as prochiral since, if either of the like substituents is converted to a different group, the tetrahedral center then becomes chiral. Consider glycerol: the central carbon of glycerol is prochiral since replacing either of the OCH2OH groups would make the central carbon chiral. Nomenclature for prochiral centers is based on the (R,S) system (in Chapter 3). To name the otherwise identical substituents of a prochiral center, imagine
increasing slightly the priority of one of them (by substituting a deuterium for a hydrogen, for example) as shown: the resulting molecule has an (S)-configuration about the (now chiral) central carbon atom. The group that contains the deuterium is thus referred to as the pro-S group. As a useful exercise, you should confirm that labeling the other CH2OH group with a deuterium produces the (R)-configuration at the central carbon, so that this latter CH2OH group is the pro-R substituent.
|
|
|
D |
|
|
HOH23C |
A |
|
|
1CHOH |
HOH2C |
CH2OH |
G D |
2C |
|
G |
D |
‘ |
' |
C |
' |
H |
OH |
‘ |
H |
OH |
1-d, 2(S )-Glycerol |
Glycerol |
(S -configuration at C-2) |
pro-S position |
|
|
|
CH |
OH |
|
|
CH OPO2– |
|
|
|
|
|
2 |
|
|
2 |
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
C |
|
H |
|
H |
|
C |
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
pro-R position |
|
|
CH2OPO32– |
|
|
CH2 OH |
|
|
|
|
L-Glycerol-3-phosphate |
D-Glycerol-1-phosphate |
sn-Glycerol-3-phosphate
O |
|
|
|
|
C |
O |
|
|
|
O |
|
CH2 |
|
|
|
|
|
|
C |
O |
C |
H |
CH3 |
|
|
|
O |
|
|
CH2 |
O P O CH2CH2 |
N+ CH3 |
Phosphatidylcholine |
O– |
CH3 |
8.3 ● Glycerophospholipids |
245 |
FIGURE 8.5 ● The absolute configuration of sn-glycerol-3-phosphate. The pro-(R) and pro- (S) positions of the parent glycerol are also indicated.
GLYCEROLIPIDS WITH OTHER HEAD GROUPS: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
+ |
|
|
|
|
|
|
|
O |
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
P |
|
O |
|
CH2CH2 |
|
|
NH3 |
|
|
|
|
O |
|
P |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phosphatidylethanolamine |
|
|
|
|
|
|
O |
H |
|
|
C |
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
COO– |
|
|
|
|
|
P |
|
O |
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
P |
|
|
O |
|
CH2 |
|
CH |
|
|
|
|
Diphosphatidylglycerol (Cardiolipin) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
O– |
NH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phosphatidylserine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
H |
HO |
OH |
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
O |
|
|
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P |
|
|
O |
|
|
CH |
|
CH2 |
|
|
|
|
O P |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
OH |
|
OH |
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phosphatidylglycerol |
|
|
|
|
|
|
|
|
Phosphatidylinositol |
FIGURE 8.6 ● Structures of several glycerophospholipids and space-filling models of phosphatidylcholine, phosphatidylglycerol, and phosphatidylinositol.
246 Chapter 8 ● Lipids
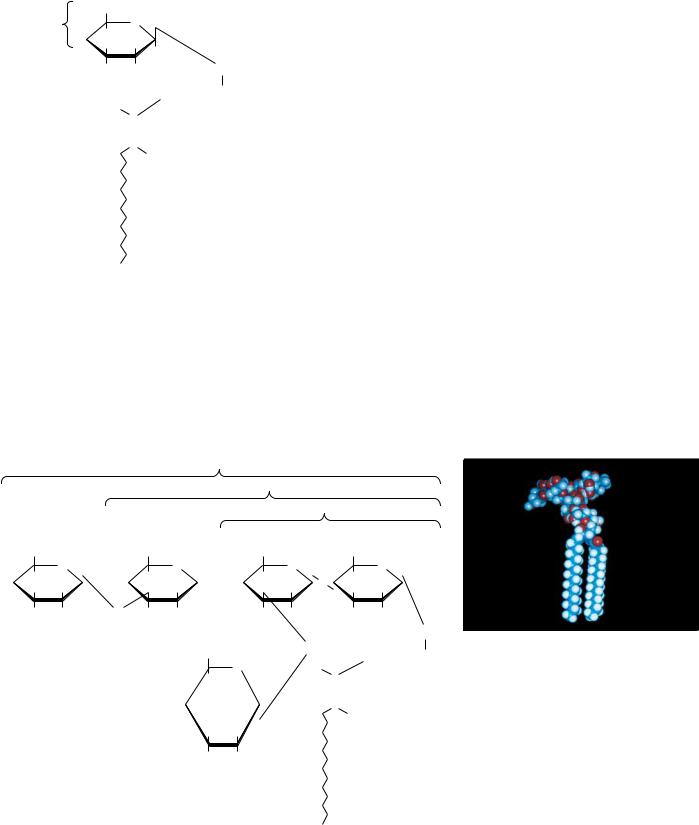
variety of polar groups are esterified to the phosphoric acid moiety of the molecule. The phosphate, together with such esterified entities, is referred to as a “head” group. Phosphatides with choline or ethanolamine are referred to as phosphatidylcholine (known commonly as lecithin) or phosphatidylethanolamine, respectively. These phosphatides are two of the most common constituents of biological membranes. Other common head groups found in phosphatides include glycerol, serine, and inositol (Figure 8.6). Another kind of glycerol phosphatide found in many tissues is diphosphatidylglycerol. First observed in heart tissue, it is also called cardiolipin. In cardiolipin, a phosphatidylglycerol is esterified through the C-1 hydroxyl group of the glycerol moiety of the head group to the phosphoryl group of another phosphatidic acid molecule.
A D E E P E R L O O K
Glycerophospholipid Degradation: One of the Effects of Snake Venoms
The venoms of poisonous snakes contain (among other things) a class of enzymes known as phospholipases, enzymes that cause the breakdown of phospholipids. For example, the venoms of the eastern diamondback rattlesnake (Crotalus adamanteus) and the Indian cobra (Naja naja) both contain phospholipase A2, which catalyzes the hydrolysis of fatty acids at the C-2 position of glycerophospholipids.
The phospholipid breakdown product of this reaction, lysolecithin, acts as a detergent and dissolves the membranes of red blood cells, causing them to rupture. Indian cobras kill several thousand people each year.
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
O |
|
P |
|
|
|
|
O |
|
P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
O |
O– |
|
|
|
|
H |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
H2O |
|
|
|
|
|
+ O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2C |
|
C CH2 |
H2C |
|
C CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eastern diamondback rattlesnake. (Dr. E. R. |
O |
|
O |
|
|
O |
HO |
Degginger) |
|
|
|
O |
O |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PLA2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Phospholipid
Indian cobra. (Dr. E. R. Degginger)
FIGURE 8.8
8.3 ● Glycerophospholipids |
247 |
FIGURE 8.7 ● A space-filling model of 1-stearoyl-2-oleoyl-phosphatidylcholine.
Phosphatides exist in many different varieties, depending on the fatty acids esterified to the glycerol group. As we shall see, the nature of the fatty acids can greatly affect the chemical and physical properties of the phosphatides and the membranes that contain them. In most cases, glycerol phosphatides have a saturated fatty acid at position 1 and an unsaturated fatty acid at position 2 of the glycerol. Thus, 1-stearoyl-2-oleoyl-phosphatidylcholine (Figure 8.7) is a common constituent in natural membranes, but 1-linoleoyl-2-palmitoylphos- phatidylcholine is not.
Both structural and functional strategies govern the natural design of the many different kinds of glycerophospholipid head groups and fatty acids. The structural roles of these different glycerophospholipid classes are described in Chapter 9. Certain phospholipids, including phosphatidylinositol and phosphatidylcholine, participate in complex cellular signaling events. These roles, appreciated only in recent years, are described in Chapter 34.
Ether Glycerophospholipids
Ether glycerophospholipids possess an ether linkage instead of an acyl group at the C-1 position of glycerol (Figure 8.8). One of the most versatile biochemical signal molecules found in mammals is platelet activating factor, or PAF, a unique ether glycerophospholipid (Figure 8.9). The alkyl group at C-1 of PAF is typically a 16-carbon chain, but the acyl group at C-2 is a 2-carbon acetate unit. By virtue of this acetate group, PAF is much more water-soluble
O
+
–O P O CH2 CH2 NH3
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
H2C |
|
|
CH |
|
|
CH2 |
|
|
|
|
|
|
|
|
Ether O |
|
|
|
|
|
|
Ester |
O |
|
|
|
linkage |
|
|
|
|
|
|
|
linkage |
R1 |
C |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
|
|
|
● A 1-alkyl 2-acyl-phos- phatidylethanolamine (an ether glycerophospholipid).
A D E E P E R L O O K
Platelet Activating Factor: A Potent Glyceroether Mediator
Platelet activating factor (PAF) was first identified by its ability (at low levels) to cause platelet aggregation and dilation of blood vessels, but it is now known to be a potent mediator in inflammation, allergic responses, and shock. PAF effects are observed at tissue concentrations as low as 10 12 M. PAF causes a dramatic inflammation of air passages and induces asthma-like symptoms in laboratory animals. Toxic-shock syndrome occurs when fragments of destroyed bacteria act as toxins and induce the synthesis of PAF. This results in a drop in blood pressure and a reduced
volume of blood pumped by the heart, which leads to shock and, in severe cases, death.
Beneficial effects have also been attributed to PAF. In reproduction, PAF secreted by the fertilized egg is instrumental in the implantation of the egg in the uterine wall. PAF is produced in significant quantities in the lungs of the fetus late in pregnancy and may stimulate the production of fetal lung surfactant, a pro- tein–lipid complex that prevents collapse of the lungs in a newborn infant.
FIGURE 8.9
248 Chapter 8 ● Lipids
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
CH3 |
|
|
–O |
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
P |
|
O |
|
CH2 |
|
CH2 |
|
N |
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2C |
|
|
CH |
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
O |
|
Platelet |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
activating factor |
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
● The structure of 1-alkyl 2-acetyl-phosphatidylcholine, also known as platelet activating factor or PAF.
than other lipids, allowing PAF to function as a soluble messenger in signal transduction.
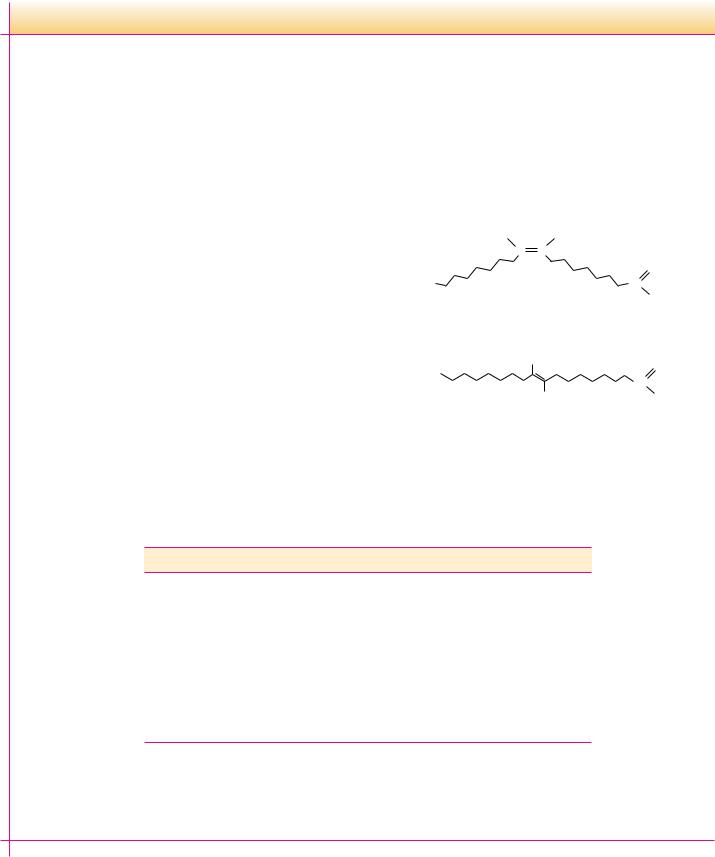
Plasmalogens are ether glycerophospholipids in which the alkyl moiety is cis- , -unsaturated (Figure 8.10). Common plasmalogen head groups include choline, ethanolamine, and serine. These lipids are referred to as phosphatidal choline, phosphatidal ethanolamine, and phosphatidal serine.
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
–O |
|
|
|
P |
|
O |
|
CH2CH2 N |
CH3 |
|
|
|
|
|
|
|
|
|
|
|
Choline plasmalogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The ethanolamine |
|
|
|
CH2 |
|
CH |
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
plasmalogens have |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ethanolamine in |
H |
|
|
O |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
place of choline. |
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H
FIGURE 8.10 ● The structure and a space-filling model of a choline plasmalogen.
|
|
|
OH |
|
H |
OH |
|
|
OH |
H |
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H2O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
C |
|
|
C |
|
CH2 |
|
|
C |
|
C |
|
|
CH2 |
|
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
C |
|
H |
NH3 |
|
|
|
|
|
|
C |
H |
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
COOH |
|
|
|
|
|
|
|
|
|
|
|
|
C |
H |
|
|
|
|
|
|
|
Fatty acid |
C |
C |
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R
8.4 ● Sphingolipids
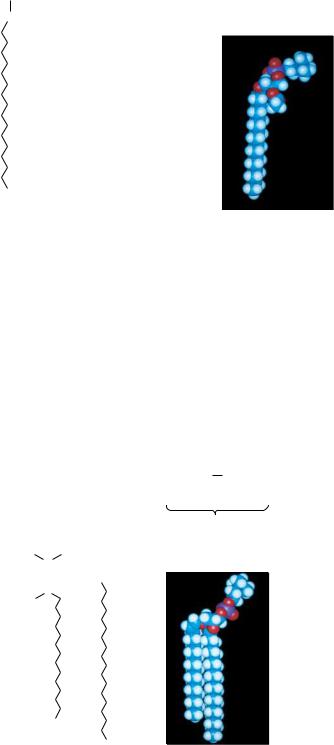
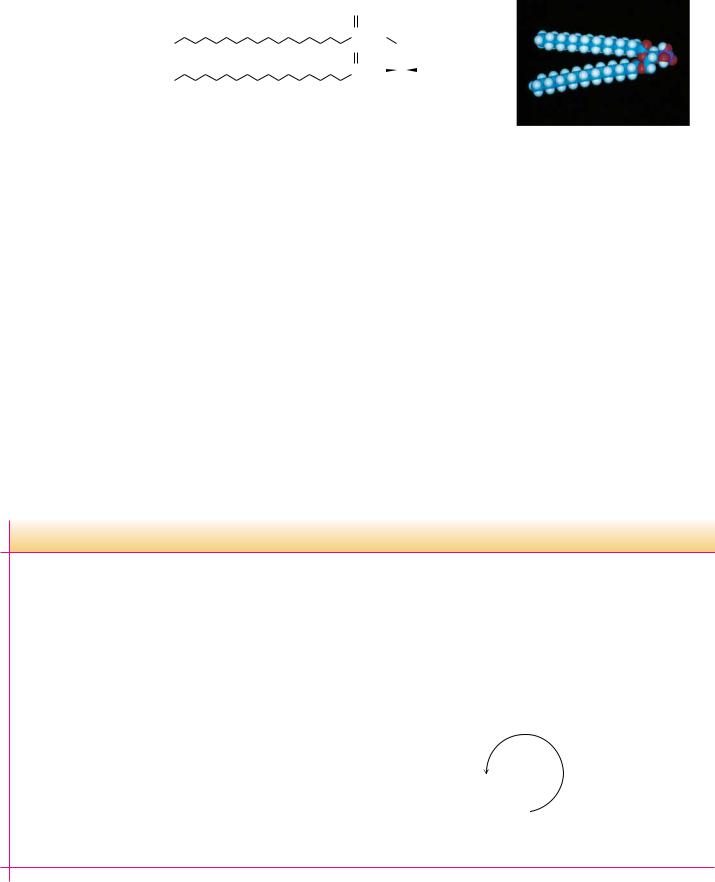
Sphingolipids represent another class of lipids found frequently in biological membranes. An 18-carbon amino alcohol, sphingosine (Figure 8.11), forms the backbone of these lipids rather than glycerol. Typically, a fatty acid is joined to a sphingosine via an amide linkage to form a ceramide. Sphingomyelins represent a phosphorus-containing subclass of sphingolipids and are especially important in the nervous tissue of higher animals. A sphingomyelin is formed by the esterification of a phosphorylcholine or a phosphorylethanolamine to the 1-hydroxy group of a ceramide (Figure 8.12).
FIGURE 8.11 ● Formation of an amide linkage between a fatty acid and sphingosine produces a ceramide.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
CH3 |
|
|
|
|
|
|
|
–O |
|
|
|
|
|
|
|
|
|
|
|
|
+ |
CH3 |
|
|
|
|
|
|
|
|
|
|
P |
|
O |
|
CH2CH2 |
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
H |
|
|
|
O |
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
C |
|
C |
|
|
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C |
H |
C |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Choline sphingomyelin with stearic acid
FIGURE 8.12 ● A structure and a space-filling model of a choline sphingomyelin formed from stearic acid.