
Multidimensional Chromatography
.pdfMultidimensional High Resolution Gas Chromatography |
69 |
The analysis of persistent organic pollutants presents particular problems to the analyst. While instrumental methods exist for determining the bulk chlorinated content, the variability in toxicity effects means that these are of little use in health impact assessment. A fully speciated analysis of each individual congener is required, and the analysis is hampered not only by target species complexity, but the more concentrated and equally complex sample matrix. In both soils and atmospheric samples, the overwhelming organic background is that of toxicologically insignificant aliphatic species. The isolation of organochlorine compounds against this background requires considerable sample preparation prior to analysis, in combination with selective detection (often using electron-capture detectors (ECDs) or MS) and a high resolution separation. Ideally, a single column would be used for the universal separation of all congeners of interest; however, after many years of optimization this seems unlikely to emerge (49, 50). Extensive multilaboratory studies of retention behaviour on a variety of stationary phases (51) have highlighted that it is not possible to determine all species under a single set of single-column conditions. A number of approaches to deconvolution have been undertaken, including the use of (i) parallel columns to increase probability of isolation on at least one column, (ii) mass spectrometric deconvolution, (iii) serially coupled columns, and (iv) two-dimensional GC. While option (iv) requires significant apparatus and further development, it is the most reliable method on offer at the present time.
Work by Kinghorn et al. has demonstrated a two-dimensional separation of the PCB, Aroclor 1254. This separation used a non-polar primary column with selected cuts to a secondary chiral selective column (52). This system utilized a combination of a Deans switch transfer with a cryogenically cooled intermediate capillary column which was used to refocus the analytes prior to secondary column analysis. Poorly resolved single-column peaks were well resolved on application of a second separation in combination with the refocusing step, with the exception being congener 138, which may be resolved only through the use of very polar cyanopropyl or liquid crystal phase columns (53, 54). Chromatograms from this work are shown later in Chapter 13 (see Figure 13.1).
Of the 209 PCB congeners, 78 are known to exist in two chiral forms. Rather than chirality based around a central carbon atom, asymmetric substitution of both phenyl rings leads to axial chirality of all non-planar conformations. Many of these have low energies of transformation and are able to interconvert by rotation about the central C – C bond and form racemic mixtures. There are however 10 chiral trior tetraorthosubstituted PCBs which have rotational energy barriers sufficiently high to be conformationally stable and thus will not undergo racemization – these are known as atropisomers. There has been recent evidence to show that under certain conditions the biodegradation of PCBs favours one enantiomer and the relative ratio of the respective enantiomers may be used to study this phenomenon (since the physicochemical and transport properties will not affect the enantiomeric ratios).
It is in the study of this phenomenon where two-dimensional GC offers by far the most superior method of analysis. The use of chiral selector stationary phases, in particular modified cyclodextrin types, allows apolar primary and atropisomer selective secondary separation. Reported two-dimensional methods have been successful
70 |
Multidimensional Chromatography |
in isolating several important pairs of atropisomers (55), and quantitation has been possible since peaks have been baseline-separated by using simple well characterized detectors such as ECDs. Examples of quantitative two-dimensional separations which have been used to identify non-racemic distributions of PCBs in river and marine samples have been reported recently (56).
Chlorinated dioxins and benzofurans are perhaps the most toxic of all persistent organic compounds found in the environment. Their toxicity is by no means universal, and variations between apparently similar molecules can vary by several orders of magnitude. Because of this specificity, analysis must be similarly species-specific. This group of compounds suffer from the common problem of being present in a matrix of hydrocarbon species at a far higher concentration. In order to simplify the separation, a very extensive sample clean-up is often required, and this can aid in gaining reasonably high resolution even on a single column. The use of two-dimen- sional techniques reduces the degree to which interfering compounds must be removed at the off-line clean-up stage, thus increasing reproducibly and reducing sample handling. Work carried out in the mid 1980s demonstrated the two-dimen- sional separation of several polychlorinated dibenzo-p-dioxins (PCDDs) congeners, and also illustrated how the period of heart-cut transfer was critical in obtaining a baseline-resolved secondary chromatogram (57). In addition, for PCDDs and PCBs, the use of ECDs is widespread. This is for a number of reasons, related to cost, ease of quantitation and sensitivity, which all favour the use of ECDs as opposed to mass spectrometric detectors. It is important, however, since identification is based on retention index alone, that the timing of heart-cuts in the primary dimension are extremely precise. Recent developments in electronic pressure and flow rate control has greatly improved this reliability.
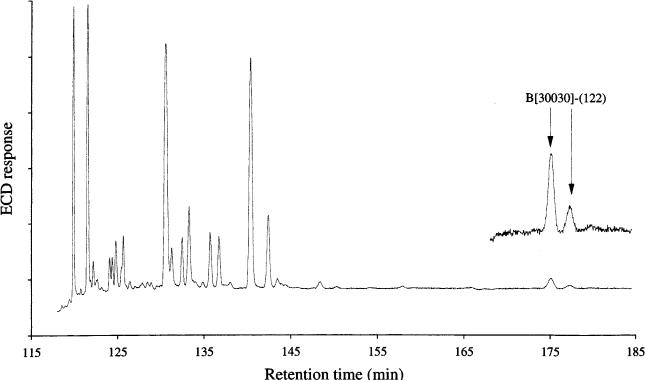
Chlorinated camphene and bornane compounds (referred to generally as toxaphenes) are a further group of anthropogenically produced persistent organic pollutants (58). While most of the 200 reported derivatives are chiral in nature, they are manufactured and subsequently occur in the environment as racemates. The degradation of such species within organisms, however, occurs non-racemically and this phenomenon may be used to study the exact metabolic processes involved in their degradation. In a similar way to PCBs, this places an analytical requirement that any determination is both isomericand enantiomeric-specific. Two-dimen- sional GC had been applied to the study of toxaphenes in marine mammal samples (59), by using an apolar primary column and a chiral secondary dimension. The chromatogram shown in Figure 3.8 illustrates several heart-cuts from the primary column, demonstrating that in many instances a non-racemic mixture of enantiomers is obtained, as a result of metabolic degradation. The separation was enabled by using non-polar and chiral columns, with independent temperature programming of both of these. While a large number of target compounds with potentially differing enantiomeric ratios were identified, each pair required a heart cut and subsequent analysis. The authors noted that although the separations were baseline, and thus only possible through this analytical route, the number and length of analyses required to characterize large number of species in a complex mixture is still a major limitation of GC– GC.
Chromatography Gas Resolution High Multidimensional
Figure 3.8 Second-dimension chiral cyclodextrin capillary column separation of a non-racemic pair of nonachlorobornane compounds extracted from dolphin blubber, shown with expanded attenuation in the inset. The primary separation (not shown) was performed on an apolar primary capillary column. Reproduced from H.-J. de Geus et al. J. High Resol. Chromatogr. 1998, 21, 39 (59).
71
72 |
Multidimensional Chromatography |
3.4CONCLUSIONS
While far less widely reported in the literature than GC – MS coupled techniques, heart-cut two-dimensional gas chromatography offers very many solutions to the analysis of trace level components in complex mixtures. The area of petrochemical analysis is likely to continue to be a source of new innovative methods for the analysis of aromatic and polynuclear aromatic compounds, as well as pioneering the isolation of heteroatom species. It is the analysis of enantiomeric species, however, where two-dimensional GC currently excels. The ability to study chiral compounds released as a result of natural processes may hold the key to a better understanding of many biological and biochemical processes, as well as giving essential insights into food, flavour and fragrance science. The ability to differentiate between atropisomers related to persistent chlorinated organic pollutants will also have a major impact on the way that we study the remediation and cycling of such species in the environment. Since two-dimensional separations already allow the isolation of many trace species at both isomeric and enantiomeric levels, it is likely that future developments and coupling to isotope-ratio mass spectrometry will also offer new possibilities in tracing the formation and fate of organic compounds in both industrial and natural processes.
REFERENCES
1.L. A. Luke and J. V. Brunnock, ‘Separation of naphthenic and paraffinic hydrocarbons
up to C11 from hydrocarbon mixtures by gas chromatography on faujasite molecular sieves’, Ger. Offen. 1 908 418 (1968).
2.J. C. Giddings, ‘Maximum number of components resolvable by gel filtration and other elution chromatographic methods’, Anal. Chem. 39: 1027 – 1028 (1967).
3.T. A. Berger, ‘Separation of a gasoline on an open tubular column with 1.3 million effective plates’, Chromatographia 42: 63 – 71 (1996).
4.Z. Liu and J. B. Phillips, ‘Comprehensive two-dimensional gas chromatography using an on-column thermal modulator interface’, J. Chromatogr. Sci. 29: 227 – 231 (1991).
5.K. Grob, Split and Splitless Injection in Capillary GC, W. Bertsch, W. G. Jennings and P. Sandra (Series Eds), Hüthig, Heidelberg, Germany (1991).
6.E. Boselli, B. Grolimund, K. Grob, G. Lercker and R. Amadò, ‘Solvent trapping during large volume injection with an early vapor exit. Part 1: description of the flooding process’, J. High Resolut. Chromatogr. 21: 355 – 362 (1998).
7.B. M. Gordon, C. E. Rix and M. F. Borgerding, ‘Comparison of state-of-the-art column switching techniques in high resolution gas chromatography’, J. Chromatogr. Sci. 23: 1 – 10 (1985).
8.P. L. Mills and W. E. Guise, ‘A multidimensional gas chromatographic method for analysis of n-butane oxidation reaction products’, J. Chromatogr. Sci. 14: 431 – 459 (1996).
9.S. T. Adam, ‘Quality test of a mechanical switching valve for two-dimensional open tubular gas chromatography’, J. High Resolut. Chromatogr. Chromatogr. Commun. 11: 85 – 89 (1988).
10.K. Shiomi, ‘Determination of acetaldehyde, acetal and other volatile congeners in alcoholic beverages using multidimensional capillary gas chromatography’, J. High Resolut. Chromatogr. Chromatogr. Commun. 14: 136 – 137 (1991).
Multidimensional High Resolution Gas Chromatography |
73 |
11.R. R. Deans, ‘A new technique for heart cutting in gas chromatography’, Chromatographia 1: 18 – 22 (1968).
12.W. Bertsch, ‘Multidimensional gas chromatography’, in Multidimensional Chromatography. Techniques and applications, H. J. Cortes (Ed.), Chromatographic Science Series, Vol. 50, Marcel Dekker, New York, pp. 74 – 144 (1990).
13.D. C. Fenimore, R. R. Freeman and P. R. Loy, ‘Determination of 9-tetrahydrocannabi- nol in blood by electron capture gas chromatography’, Anal. Chem. 45: 2331 – 2335 (1973).
14.H. J. Neumann, B. Paczynska-Lahme and D. Severin, Geology of Petroleum, Vol. 5, Composition and Properties of Petroleum, Ferdinand Enke, Stuttgart Germany (1981).
15. H. Boer and P. van Arkel, ‘Automatic PNA (paraffin-naphthene-aromatic) analyzer for (heavy) naphtha’, Chromatographia 4: 300 – 308 (1971).
16.P. van Arkel, J. Beens, H. Spaans, D. Grutterink and R. Verbeek, ‘Automated PNA analysis of naphthas and other hydrocarbon samples’, J. Chromatogr. Sci. 25: 141 – 148 (1988).
17.P. Coleman and L. S. Ettre, ‘Analysis of gases containing inorganic and organic compounds using a combination of a thick-film capillary column and a packed adsorption column’, J. High Resolut. Chromatogr Chromatogr. Commun. 8: 112 – 118 (1985).
18.H. Tani and M. Furuno, ‘Rapid analysis of hydrocarbons and inert gases by a multidimensional gas chromatograph’, J. High Resolut. Chromatogr. Chromatogr. Commun. 9: 712 – 716 (1985).
19.S. Wu, W. H. Chatham and S. O. Farwell, ‘Multidimensional HRGC for sample components with a wide range of volatilities and polarities’, J. High Resolut. Chromatogr. 13: 229 – 233 (1990).
20.R. G. Schäfer and J. Höltkemeier, ‘Determination of dimethylnaphthalenes in crude oils by means of two-dimensional capillary gas chromatography’, Anal. Chim. Acta 260: 107 – 112 (1992).
21.J. High Resol. Chromatogr, 17 (4 and 6) (1994).
22.J. J. Szakasits and R. E. Robinson, ‘Hydrocarbon type determination of naphthas and catalytically reformed products by automated multidimensional gas chromatography’, Anal. Chem. 63: 114 – 120 (1991).
23.B. M. Gordon, M. S. Uhrig, M. F. Borgerding, H. L. Chung, W. M. Coleman, J. F. Elder,
J.A. Giles, D. S. Moore, C. E. Rix and E. L. White, ‘Analysis of flue-cured tobacco essential oil by hyphenated analytical techniques’, J. Chromatogr. Sci. 26: 174 – 180 (1988).
24.A. C. Lewis, K. D. Bartle and L. Rattner, ‘High-speed isothermal analysis of atmospheric isoprene and DMS using online two-dimensional gas chromatography’,
Environ. Sci. Technol. 31: 3209 – 3217 (1997).
25.S. Blomberg and J. Roeraade, ‘Preparative capillary gas chromatography. II. Fraction collection on traps coated with a very thick-film of immobilized stationary phase’,
J.Chromatogr. 394: 443 – 453 (1987).
26.J. P. E. M. Rijks and J. A. Rijks, ‘Programmed cold sample introduction and multidimensional preparative capillary gas chromatography. Part I: introduction, design and
operation of a new mass flow controlled multidimensional GC system’,
J. High Resolut. Chromatogr. 13: 261 – 266 (1990).
27.O. Nishimura, ‘Application of a thermal desorption cold trap injector to multidimensional GC and GC – MS’, J. High Resolut. Chromatogr. 18: 699 – 704(1995).
28.R. H. M. van Ingen and L. M. Nijssen, ‘Determination of diethylene glycol monoethyl ether in flavors by two-dimensional capillary gas chromatography’, J. High Resolut. Chromatogr. 12: 484 – 485 (1989).
29.S. Nitz, F. Drawert, M. Albrecht and U. Gellert, ‘Micropreparative system for enrichment of capillary GC effluents’, J. High Resolut. Chromtogr. 11: 322 – 327 (1988).
74 |
Multidimensional Chromatography |
30.D. W. Wright, K. O. Mahler and L. B. Ballard, ‘The application of an expanded multidimensional GC system to complex fragrance evaluations’, J. Chromatogr. Sci. 24: 60 – 65 (1986).
31.M. Herraiz, G. Reglero, T. Herraiz and E. Loyola, ‘Analysis of wine distillates made from muscat grapes (Pisco) by multidimensional gas chromatography and mass spectrometry’, J. Agric. Food Chem. 38: 1540 – 1543 (1990).
32.P. A. Rodriguez and C. L. Eddy, ‘Use of a two-dimensional gas chromatograph in the organoleptic evaluation of an orange extract’, J. Chromatogr. Sci. 24: 18 – 21 (1986).
33.Frank H, G. J. Nicholson and E. Bayer, ‘Rapid gas chromatographic separation of amino acid enantiomers with a novel chiral stationary phase’, J. Chromatogr. Sci. 15: 174 – 176 (1977).
34.C. Wang, F. Hartmut, G. Wang, L. Zhou, E. Bayer and P. Lu, ‘Determination of amino acid enantiomers by two-column gas chromatography with valveless column switching’, J. Chromatogr. 262: 352 – 359 (1983).
35.L. Mondello, M. Catalfamo, P. Dugo and G. Dugo, ‘Multidimensional capillary GC – GC for the analysis of real complex samples. Part II. Enantiomeric distribution of monoterpene hydrocarbons and monoterpene alcohols of cold-pressed and distilled lime oils’, J. Microcolumn Sep. 10: 203 – 212 (1998).
36.L. Mondello, M. Catalfamo, A. R. Proteggente, I. Bonaccorsi and G. Dugo, ‘Multidimensional capillary GC – GC for the analysis of real complex samples. 3. Enantiomeric distribution of monoterpene hydrocarbons and monoterpene alcohols of mandarin oils’, J. Agric. Food Chem. 43: 54 – 61 (1998).
37.A. Mosandl, K. Fischer, U. Hener, P. Kreis, K. Rettinger, V. Schubert and H.-G. Schmarr, ‘Stereoisomeric flavor compounds. 48. Chirospecific analysis of natural flavors and essential oils using multidimensional gas chromatography’, J. Agric. Food Chem. 39: 1131 – 1134 (1991).
38.G. Full, P. Winterhalter, G. Schmidt, P. Herion and P. Schreier, ‘MDGC – MS: a powerful tool for enantioselective flavor analysis’, J. High Resolut. Chromatogr. 16: 642 – 644 (1993).
39.A. Mosandl, U. Hener, U. Hagenauer-Hener and A. Kustermann, ‘Direct enantiomer separation of chiral -lactones from food and beverages by multidimensional gas chromatography’, J. High Resolut. Chromatogr. 12: 532 – 536 (1989).
40.D. Häring, T. König, B. Withopf, M. Herderich and P. Schreier, ‘Enantiodifferentiation
of -ketols in sherry by oneand two-dimensional HRGC techniques’, J. High Resolut. Chromatogr. 20: 351 – 354 (1997).
41.W. A. König, B. Gehrcke, D. Ischeln, P. Evers, J. Dönnecke and W. Wang, ‘New selectively substituted cyclodextrins as stationary phases for the analysis of chiral constituents of essential oils’, J. High Resolut. Chromatogr. 15: 367 – 372 (1992).
42.C. Bicchi and A. Pisciotta, ‘Use of two-dimensional gas chromatography in the direct
enantiomer separation of chiral essential oil components’, J. Chromatogr. 508: 341 – 348 (1990).
43.C. Askari, U. Hener, H-G. Schmarr, A. Rapp and A. Mosandl, ‘Stereodifferentiation of some chiral monoterpenes using multidimensional gas chromatography’, Fresenius J. Anal. Chem. 340: 768 – 772 (1991).
44.V. Karl, H-G. Schmarr and A. Mosandl, ‘Simultaneous stereoanalysis of 2-alkyl-branched acids, esters and alcohols using a selectivity-adjusted column system in multidimensional gas chromatography’, J. Chromatogr. 587: 347 – 350 (1991).
45.A. Kaunzinger, F. Podebrad, R. Liske, B. Maas, A. Dietrich and A. Mosandl, ‘Stereochemical differentiation and simultaneous analysis of 3-,4- and 5-hydroxyalkanoic
Multidimensional High Resolution Gas Chromatography |
75 |
acids from biopolyesters by multidimensional gas chromatography’, J. High Resolut. Chromatogr. 18: 49 – 53 (1995).
46.S. Nitz, B. Weinreich and F. Drawert, ‘Multidimensional gas chromatography – isotope ratio mass spectrometry (MDGC – IRMS). Part A: system description and technical requirements’, J. High Resolut. Chromatogr. 15: 387 – 391 (1992).
47.D. Juchelka, T. Beck, U. Hener, F. Dettmar and A. Mosandl, ‘Multidimensional gas chromatography coupled on-line with isotope ratio mass spectrometry (MDGC – IRMS): progress in the analytical authentication of genuine flavor components’, J. High Resolut. Chromatogr. 21: 145 – 151 (1998).
48.G. M. Frame, ‘A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. Part 2. Semi-quantitative Aroclor congener distributions’, Anal. Chem. 70: 714 – 722 (1997).
49.B. R. Larsen, ‘HRGC separation of PCB congeners’, J. High Resolut. Chromatogr. 18: 141 – 151 (1995).
50.P. Hess, J. de Boer, W. P. Cofino, P. E. G. Leonards and D. E. Wells, ‘Critical review of the analysis of nonand mono-ortho-chlorobiphenyls’, J. Chromatogr. 703: 417 – 465 (1995).
51.G. M. Frame, ‘A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. Part 1. Retention and coelution database’, Fresenius’ J. Anal. Chem. 357: 701 – 713 (1997).
52.R. M. Kinghorn, P. J. Marriott and M. Cumbers, ‘Multidimensional capillary gas chromatography of polychlorinated biphenyl marker compounds’, J. High Resolut. Chromatogr. 19: 622 – 626 (1996).
53.J. de Boer and Q. T. Dao, ‘Analysis of seven chlorobiphenyl congeners by multidimensional gas chromatography’, J. High Resolut. Chromatogr. 14: 593 – 596 (1991).
54.J. de Boer and Q. T. Dao, ‘Interferences in the determination of 2,4,5,2 ,5 - pentachlorobiphenyl (CB 101) in environmental and technical samples’, Int. J. Environ. Anal. Chem. 43: 245 – 251 (1991).
55.E. Benická, R. Novakovsky, J. Hrouzek, J. Krupcík, P. Sandra and J. de Zeeuw, ‘Multidimensional gas chromatographic separation of selected PCB atropisomers in technical formulations and sediments’, J. High Resolut. Chromatogr. 19: 95 – 98 (1996).
56.G. P. Blanch, A. Glausch, V. Schurig, R. Serrano and M. J. Gonzalez, ‘Quantification and determination of enantiomeric ratios of chiral PCB 95, PCB 132 and PCB 149 in shark liver samples (C. coelolepis) from the Atlantic ocean’, J. High Resolut. Chromatogr. 19: 392 – 396 (1996).
57.G. Schomburg, H. Husmann and E. Hübinger, ‘Multidimensional separation of isomeric species of chlorinated hydrocarbons such as PCB, PCDD and PCDF’, J. High Resolut. Chromatogr. Chromatogr. Commun. 8: 395 – 400 (1985).
58.D. Hainzl, J. Burhenne and H. Parlar, ‘HRGC – ECD and HRGC – NICI SIM quantification of toxaphene residues in selected marine organism by environmentally relevant chlorobornanes as standard’, Chemosphere 28: 237 – 243 (1994).
59.H.-J. de Geus, R. Baycan-Keller, M. Oehme, J. de Boer and U. A. Th Brinkman, ‘Determination of enantiomer ratios of bornane congeners in biological samples using heart-cut multidimensional gas chromatography’, J. High Resolut. Chromatogr. 21: 39 – 46 (1998).

Multidimensional Chromatography
Edited by Luigi Mondello, Alastair C. Lewis and Keith D. Bartle
Copyright © 2002 John Wiley & Sons Ltd
ISBNs: 0-471-98869-3 (Hardback); 0-470-84577-5 (Electronic)
4 Orthogonal GC–GC
P. J. MARRIOTT
Royal Melbourne Institute of Technology, Melbourne, Australia
4.1 INTRODUCTION TO MULTIDIMENSIONAL GAS CHROMATOGRAPHY
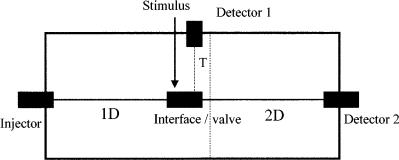
A multidimensional gas chromatography (MDGC) separation involves two columns in the separation process. However, simply the joining together of two columns is not sufficient to produce the MDGC process. The direct coupling of two different columns – called multichromatography (1) – is able to improve separation, but is essentially the same as mixing stationary phases (in this case, the two phases on the coupled columns) to obtain an improved selectivity in the separation. In Figure 4.1, the interface or valve at the confluence of the two columns is simply a column join in this instance. There is no increase in capacity (total number of available separation plates) in the system, but merely a shifting of the peak relative retentions. This experiment has been improved in its implementation by locating a valve between the two columns, thus allowing the mid-point pressure to be altered (the stimulus for the valve in Figure 4.1 is variable pressure). This is termed a pressure-tuning experiment (2), and by varying the pressure the relative contribution of each column to the separation can be varied, and so a wide range of apparent retention factors can be obtained. By optimization of the pressure and other experimental settings (3, 4), the chromatographic separation can be consequently optimized according to the mixture being studied. This method still does not qualify to be called MDGC. The MDGC definition must be cast to allow differentiation between the accepted MDGC experiment and the above coupled-column approaches. It must incorporate the process of isolation of zones of effluent from column 1 (also called dimension 1 (1D)) and then passing them to column 2 (also called dimension 2 (2D)). One could believe that this must involve extra-column couplings, but that has been obviated by recent developments (see below). Thus, the simplest MDGC arrangement will be to use a valve switching system that can pass zones of effluent from 1D to 2D. This will be the subject of other chapters in this book and so will not be treated any further here. In the case of the system shown in Figure 4.1, the interface or valve will be a mechanical valve or a pressure switching valve that can be switched to pass effluent from 1D to 2D, while the stimulus will be the electronic valve drive or balancing pressure supply. Those zones not passed to 2D will be directed to the detector at the end of 1D.
78 |
Multidimensional Chromatography |
Figure 4.1 Schematic diagram of a coupled column system. The first column (1D) is connected to the second column (2D) through the interface or valve system. The interface can be a direct coupling, a live T-union, a complex multiport valve, or a thermal or cryogenic modulation system. The stimulus can be the switching of the valve, a balancing pressure to divert flow towards 2D, an added flow that is used in pressure tuning, or the drive mechanism for the modulator. The line to detector 1 will normally be a non-retaining section of column. In a twooven system, 1D and 2D will be in different ovens; the dotted line indicates separately heated zones.
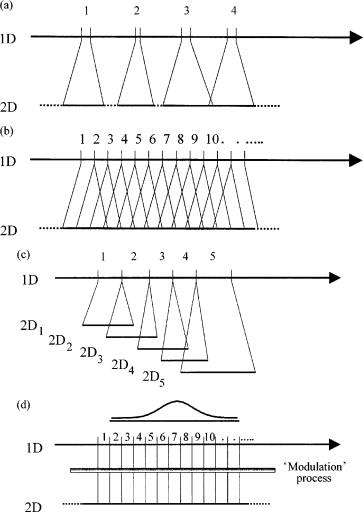
Figure 4.2(a) shows a schematic diagram of how the result might be viewed, with sections 1–4 transferred from 1D to 2D by what we call a heartcut operation. In the simplest mode, 2D can be a column which continues the chromatographic process immediately the zone is passed to it, or it can incorporate a cryotrap or cooled zone which focusses or traps all of the segments at the start of 2D before they are allowed to travel along the second column. The latter can be located in a second oven, such as is shown by the dotted line separating the two sections in Figure 4.1.
The separation zone on 1D has been expanded on 2D, and so if peaks are not resolved on 1D their separation on 2D will be achieved provided that the selectivity of the latter towards the specific components being transferred allows this. The total peak capacity is increased because we add the number of plates (or separable peaks) from 1D to that of 2D. In some cases, the components from the 1D heartcuts may overlap on 2D. This will reduce the total resolution. It is logical that such overlap should be minimal if we are to prevent solutes from different zones from overlapping on the subsequent 2D analysis. If the argument is extended to the limit, we get the situation shown in Figure 4.2(b) where every small zone of 1D is cut to 2D. The final result will almost be equivalent to just analysing the sample on 2D (or equivalent to the multichromatography method). Hence, MDGC with heartcuts has an inherent problem where many separate parts of the 1D analysis are of interest in respect of the analyst wanting to increase their resolution or separation.
The conventional MDGC experiment is essentially a limited multidimensional separation method, applying the advantage of MDGC to only limited portion(s) of a separation problem. Thus, we might conclude that MDGC can be applied to either relatively simple samples, or only to small zones of a complex sample. Hence, this could be construed as not achieving the overall goal of the multidimensional
Orthogonal GC–GC |
79 |
Figure 4.2 Examples of multidimensional gas chromatography separation arrangements: (a) small heartcut zones 1 – 4 are transferred to the second column and run together on this analytical column, where ideally greater selectivity sees the zones more spread-out on the latter column; (b) if contiguous zones from 1D are all simply passed to 2D, then we get severely overlapping zones, and as a result this will be similar to just analysing the sample on the second column.(c) the contiguous zones 1 – 5 are transferred to separate traps, and each can be independently analysed in the second dimension, thus giving rise to five 2D chromatograms;
(d) if we implement the right modulation process on small contiguous zones from 1D, which is rapid with respect to the peak width times, between 1D and 2D, preferably with zone compression and fast GC conditions on 2D, then we can obtain the GC GC technique.
