
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 123
aniline, or reactions of morpholine with chlorotoluene, catalyst loadings of 0.05 % gave complete conversion and high yields. As for hindered monodentate ligands discussed below, reactions of chloropyridines with amines occurred, thus demonstrating that chelation is not necessary for coupling of this class of electrophile. However, reaction temperatures of 110 C, which are significantly higher than those needed for aryl chlorides, were necessary for reaction of these heterocyclic substrates. Finally, these ligands generated catalysts that induced clean chemistry with substrates containing base-sensitive groups. For these substrates and this ligand system, K3PO4 was an e ective base.
A variety of aryl bromides also reacted with amines at room temperature. Nevertheless, reactions at 80 C were generally the most suitable for catalyst loadings of 0.5 mol %. In general, reactions of secondary amines or of primary amines with ortho-substituted bromoarenes were most favorable. The two examples of primary alkylamines reacting with unhindered aryl halides required long reaction times or higher catalyst loadings. Reaction of benzylamine with 3,5-dimethylbromobenzene required a reaction time of 42 h, while reaction of butylamine with bromobenzonitrile required 3 mol % catalyst and 22 h. In this case, small amounts of the diarylamine were also formed. Aryl triflates were also suitable substrates and reacted with similar scope. Reactions at room temperature were conducted with NaOtBu as the base, while reactions at 80 C were conducted with K3PO4. Most recently, conditions have been optimized for reactions of aryl iodides. In this case, the dicyclohexyl ligands 13 and 14, as well as Xantphos [159], provided the best yields between room temperature and 100 C [160]. Despite some remaining limitations, these studies showed the broad scope of reactivity generated by catalysts with sterically hindered alkylphosphine ligands.
4.3.3.4 Phenyl Backbone-Derived P,O Ligands
A related set of ligands was discovered from a screening study conducted at Symyx Technologies by Guram et al. They found that phenyl backbone-derived P,O and P,N ligands were e ective for amination chemistry and that a cyclohexylphosphino version of these ligands, 17, gave high yields in the amination of aryl chlorides with secondary amines at 105 C [161, 162]. The ligand 16 in Eq. (16) provided good yields of tertiary amines from aryl bromides and acyclic secondary amines. Simply replacing the diphenylphosphino group with a dicyclohexylphosphino group, as in ligand 17, generated catalysts that are similar in activity to those bearing the cyclohexylphosphino biphenyl ligands 13 and 14. Unactivated aryl chlorides reacted with secondary cyclic or acyclic amines in good yields, and unactivated ortho-substituted aryl chlorides reacted with primary arylor alkylamines in good yields when this ligand was used in combination with Pd(dba)2. Reactions of primary amines with unhindered aryl halides were not reported.
ð16Þ

124 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
4.3.3.5 Low-Temperature Reactions Conducted with P(tBu)3 as a Ligand
As discussed above, the group at Tosoh reported high turnover numbers for the arylation of piperazine at high temperatures when an excess of P(tBu)3 was used. During preliminary kinetic studies on this process, it was discovered that the reactions occurred under much milder conditions when the isolated Pd(0) complex was used as catalyst and under even milder conditions when a 1:1 ratio of ligand to Pd(dba)2 was used [163]. Moreover, this catalyst system gave essentially quantitative yields with all secondary amines tested, including acyclic dialkylamines. Aryl chlorides were also suitable substrates, and in all cases this catalyst system provided high yields at 70 C for reactions of secondary amines or anilines. Primary amines reacted at 100–110 C [164]. In some cases, including an example of an unactivated aryl halide, room temperature reactions were observed with aryl chlorides. The 1:1 ratio of ligand to palladium, low molecular weight of the ligand, and simple structure (and therefore cost) make this catalyst system an economical one for large-scale synthesis. This ligand is more air-sensitive than the biphenylyl ligands 13–15, but is available as a 10 % solution in hexanes in a Sure/Seal bottle from Strem. Several examples of coupling reactions conducted with this ligand are shown in Table 3.
4.3.3.6 Heterocyclic Carbenes as Ligands
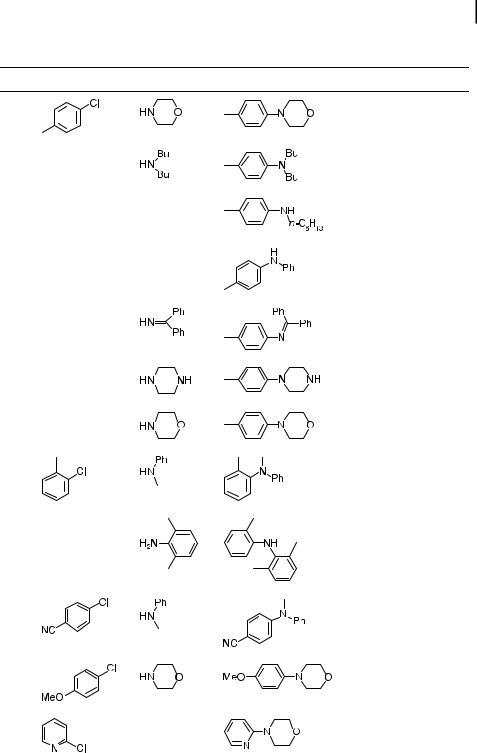
A large body of literature has recently emerged on the use of heterocyclic carbenes as ligands for many catalytic processes, and amination is no exception. These compounds can be made in a sterically hindered form, and they are strongly electron-donating. Chart 2 shows several ligand structures that have been used for palladium-catalyzed amination. In general, these ligands are added to the reaction mixture in their air-stable protonated form, and the base present in the system leads to deprotonation and subsequent coordination of the neutral carbene to palladium. These ligands are commercially available as the protonated precursors. For the amination of aryl halides, the 2,6-diisopropylphenyl-substituted carbene with a saturated backbone (18) is the most active.
Nolan reported the use of the 2,6-diisopropylphenyl imidazolium carbene precursor, which contains an unsaturated backbone, for the reaction of aryl chlorides with a variety of amines at 100 C [165, 166]. This temperature is lower than those conventionally used for reactions of aryl chlorides, but is higher than those used with P(tBu)3 or the 2-biphenylyl di-tert-butylphosphines. Reaction yields were high when 2 mol % palladium was used. Reactions of primary amines occurred in good yield, even when unhindered aryl halides were used. The monoarylamine was obtained in 86 % yield, and only a 5 % yield of the diarylamine by-product was isolated. Notably, reactions of both aryl bromides and iodides proceeded at room temperature.
Subsequently, as part of an e ort to analyze ligand libraries in a high-throughput fashion, Hartwig and co-workers showed that the carbene precursor with 2,6-diisopropyl- phenyl groups and a saturated backbone 18 generated complexes that catalyzed the reactions of aryl chlorides at room temperature and with high turnover numbers at elevated temperatures [167]. As for the catalysts formed from imidazolium salts, the ligand precursor is stable to air, simple to prepare, and is now available from Strem. In fact, complete conversion was observed for a reaction conducted without degassing the toluene solvent. These reactions are summarized in Table 4.
In general, reactions of secondary amines and of anilines occurred in high yields with
|
4.3 |
Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 125 |
||
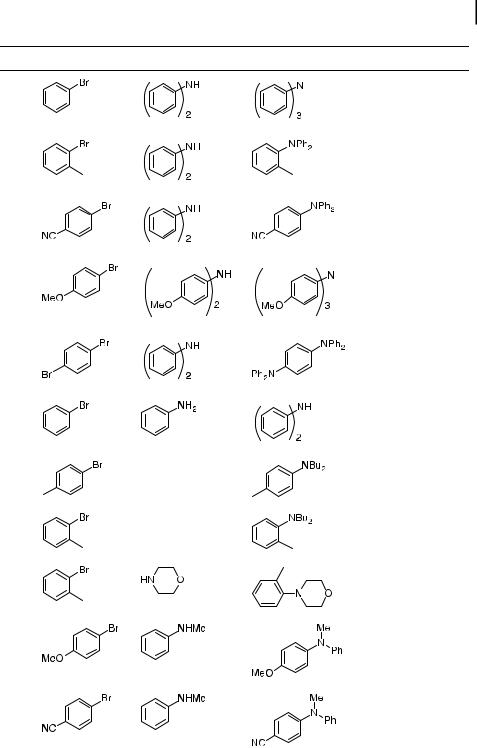
Tab. 4.3. Reactions of aryl bromides and chlorides with amines catalyzed by Pd(0)/P(t-Bu)3 |
|
|||
Entry Aryl Bromide |
Amine |
Product |
Cond.a |
Yieldb (%) |
1 |
|
|
RT |
91 |
|
|
|
1 % Pd |
|
|
|
|
1 h |
|
2 |
|
|
RT |
97 |
|
|
|
1 % Pd |
|
|
|
|
4 h |
|
3 |
|
|
RT |
97 |
|
|
|
1 % Pd |
|
|
|
|
1 h |
|
4 |
|
|
RT |
94 |
|
|
|
1 % Pd |
|
|
|
|
1 h |
|
5 |
|
|
RT |
85 |
|
|
|
1 % Pd |
|
|
|
|
1 h |
|
6 |
|
|
RT |
87 |
|
|
|
1 % Pd |
|
|
|
|
1 h |
|
7 |
HNBu2 |
RT |
90 |
|
|
|
|
1 % Pd |
|
|
|
|
4 h |
|
8 |
HNBu2 |
RT |
81 |
|
|
|
|
2 % Pdc |
|
|
|
|
6 h |
|
9 |
|
|
RT |
96 |
|
|
|
1 % Pdc |
|
|
|
|
6 h |
|
10 |
|
|
RT |
99 |
|
|
|
1 % Pd |
|
|
|
|
6 h |
|
11 |
|
|
RT |
95 |
|
|
|
1 % Pd |
|
|
|
|
5 h |
|
126 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates |
|
|
|||
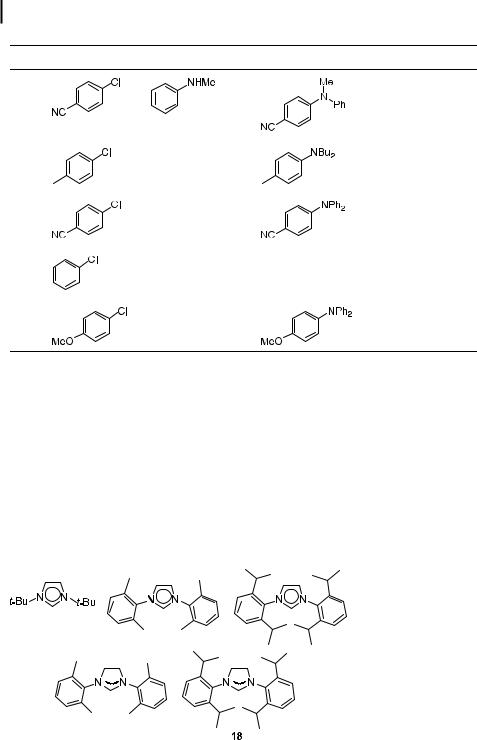
Tab. 4.3. |
(continued) |
|
|
|
|
Entry |
Aryl Bromide |
Amine |
Product |
Cond.a |
Yieldb (%) |
12 |
|
|
|
1 % Pd |
90 |
|
|
|
|
RT |
|
|
|
|
|
12 h |
|
13 |
|
HNBu2 |
|
1 % Pd |
88 |
|
|
|
|
70 C |
|
|
|
|
|
12 h |
|
14 |
|
HNPh2 |
|
1 % Pd |
89 |
|
|
|
|
RT |
|
|
|
|
|
5.5 h |
|
15 |
|
H2NPh |
HNPh2 |
5 % Pd |
75 |
|
|
|
|
RT |
|
|
|
|
|
25 h |
|
16 |
|
HNPh2 |
|
4 % Pd |
80 |
|
|
|
|
70 C |
|
|
|
|
|
16 h |
|
a Reactions run with 1 mmol of aryl halide in 1–2 mL of toluene at room temperature. Pd(dba)2 used in combination with 0.8 equiv of ligand/Pd.
b Isolated yields are an average of at least two runs. c Pd(OAc)2 used in place of Pd(dba)2.
1 mol % palladium. Even electron-rich substrates such as 4-chloroanisole gave nearly quantitative yields. As mentioned in Section 4.3.2.3, pyridyl halides can behave di erently to aryl halides because of their ability to coordinate to palladium and displace the phosphine ligands. However, the high binding energy of these heterocyclic carbene ligands [168] apparently prevents displacement of the ligand in the active form of the catalyst. Both 2- and 3- chloropyridine give complete conversion. Reactions at 100 C provide high turnover num-
Chart 2
4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 127
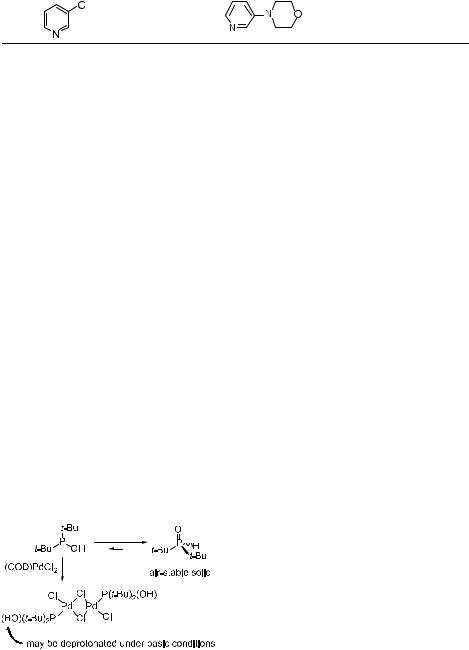
Tab. 4.4. |
|
|
Pd dba |
2 |
=18 |
|
|
|
|
|
NHRR0 |
Ar-NRR0 |
|
|
|||
|
ArCl |
ð |
Þ |
|
|
|
||
|
1:0 mmol þ |
|
|
|
|
|
||
|
1:2 mmol |
1:5 eq NaOt-Bu; DME! |
|
|
|
|||
entry ArCl |
amine |
product |
|
|
T/þC |
time |
yieldb |
|
1 |
|
|
|
|
|
rt |
3 h |
quant. |
2 |
|
|
|
|
|
rt |
20 h |
86 %c |
3 |
NH2-n-C6H13 |
|
|
|
70 |
8 h |
40 %c |
|
4 |
H2NaPh |
|
|
|
rt |
18 h |
82 %c,d |
|
5 |
|
|
|
|
|
55 |
18 h |
94 %c |
6 |
|
|
|
|
|
100 |
3 h |
71 %c,e |
7 |
|
|
|
|
|
100 |
7 h |
quant.f |
8 |
|
|
|
|
|
rt |
16 h |
97 %c |
9 |
|
|
|
|
|
rt |
4 h |
88 % |
10 |
|
|
|
|
|
rt |
3 h |
97 % |
11 |
|
|
|
|
|
rt |
5 h |
96 % |
12 |
|
|
|
|
|
rt |
3 h |
98 % |
128 |
4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates |
|
|
|
|||||
|
|
Tab. 4.4. |
(continued) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
entry |
ArCl |
amine |
product |
T/þC |
time |
yieldb |
|
13 |
|
|
|
|
rt |
20 h |
93 %c |
||
|
|
|
|
||||||
|
|
|
|
||||||
a performed at 1 mol % Pd/2 (1/1) and 1.0 M unless indicated otherwise; see [22] for furthe details.
b isolated yields are average of at least two runs; products > 95 % pure as judged by 1H NMR and GC.
c 2 mol % Pd/2.
d substrate conc. 2.0 M. e 4 equiv of amine.
f substrate conc. 2.9 M, 0.02 mol % Pd and 0.08 mol % ligand 2.
bers. Morpholine reacts with 4-chlorotoluene in quantitative yield when using 0.02 mol % catalyst. These 5000 turnover numbers are equal to the highest obtained for the amination of aryl chlorides. Piperazine, however, reacts more sluggishly, and a temperature of 100 C was necessary even when 2 mol % of catalyst was used. Reactions of primary amines were slower than those of secondary amines, and low conversions were observed at room temperature. In addition, Caddick and Cloke [169] have recently reported the use of isolated bis(1,3-di-N- tert-butylimidazol-2-ylidine)palladium(0) (see Chart 2) as a catalyst for the reactions of 4- chlorotoluene with morpholine and piperidine to give yields of 95 % and 70 %, respectively, at 100 C.
4.3.3.7 Phosphine Oxide Ligands
A clever ligand design that makes use of phosphine oxides and their tautomeric phosphinous acids has recently been reported by Li at DuPont [170, 171]. As shown in Chart 3, the coordinated di-tert-butylphosphinous acid becomes a strongly donating ligand when deprotonated, and the ligand is air-stable when free in solution. This strategy generated catalysts for a variety of cross-coupling reactions, including aryl halide amination. Although the substrate scope was not demonstrated to be particularly broad, and substrates that cannot be tackled e ectively with other ligands were not studied, the approach to ligand design was a significant departure from conventional systems.
Chart 3

4.4 Aromatic C--N Bond Formation with Non-Amine Substrates and Ammonia Surrogates 129
Chart 4
4.3.4
Heterogeneous Catalysts
Three papers have appeared in the past two years on catalysts that are either supported on polymers or are heterogeneous. Djakovitch first reported amination reactions catalyzed by palladium particles immobilized on metal oxide supports, as well as by palladium complexes contained in NaY zeolites [172]. In most cases, these reactions were conducted at high temperatures, generally 135 C. When NaOtBu was used as the base, competing amination through a benzyne intermediate was observed. Thus, para:meta regioselectivity was not high, and reaction yields were modest.
Lipshutz has reported nickel on carbon catalysts for the amination of aryl chlorides [173]. The reactions were conducted with added DPPF as the ligand. The scope of the process is similar to that seen with homogeneous nickel species. Secondary amines provide good yields with electron-poor or electron-rich aryl chlorides, and anilines are suitable for coupling with a range of aryl chlorides.
In addition, Buchwald has reported polymer-bound versions of the biphenylyl dialkylphosphines. As shown in Chart 4, the ligand is tethered to Merrifield resin through a 20-OH on the biphenylyl group. The scope for reactions was similar to that of homogeneous catalysts bearing these types of ligands. Catalysts were recycled four times starting with 2 mol % catalyst, and they largely retained their activity.
4.4
Aromatic CxN Bond Formation with Non-Amine Substrates and Ammonia Surrogates
The scope of the aromatic CaN bond formation extends beyond simple amine substrates. For example, selected imines, sulfoximines, hydrazines, lactams, azoles, carbamates, and amides all give useful products following aromatic CaN bond formation. In addition, allylamine undergoes arylation, providing an alternative to the ammonia surrogates benzylamine, tert-butyl carbamate, and benzophenone imine. Although it is an amine substrate, the reaction of this reagent is included here because of its special purpose.
ð17Þ

130 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
ð18Þ
4.4.1
Amides, Sulfonamides, and Carbamates
The initial reports on the arylation of amides and sulfonamides involved intramolecular examples and monodentate triarylphosphine ligands [100]. A paper has recently appeared that reports improved yields for intramolecular reactions when are used MOP, DPEphos, or Xantphos are used as the ligand [174] (Eqs. (17) and (18)). In general, Cs2CO3 was used for the cyclization of acetamides and carbamates, but K2CO3 proved to be the best base for the cyclization of benzamides. In the initial report, P(furyl)3 and P(o-C6H4Me)3 were the ligands that gave the most e ective catalysts. Fiveand six-membered rings, but not seven-mem- bered rings, could be formed with acceptable yields. The more recent report improved upon the original procedures, extended the chemistry to carbamates, and included the formation of five-, six-, and seven-membered rings with benzamides, acetamide, N-carbobenzyloxy, and tert-butyl carbamate substituents tethered to aryl halides, as shown in Eq. (18). The optimal ligand depended on the particular substrate.
ð19Þ
In addition, the coupling of lactams with aryl halides has been conducted in certain cases using arylphosphines, in this case DPPF. Shakespeare showed that a combination of Pd(OAc)2 and DPPF formed N-aryl lactams in good yields from five-membered lactams (Eq. (19)) [175]. Reactions involving electron-neutral aryl halides required long reaction times, but did proceed in good yield. Four-, six-, and seven-membered lactams gave poor yields with unactivated aryl halides, but good yields with electron-poor aryl halides. No reaction was, of course, observed in the absence of catalyst.
ð20Þ

4.4 Aromatic C--N Bond Formation with Non-Amine Substrates and Ammonia Surrogates 131
ð21Þ
Most recently, significant improvements have been made with regard to the reaction of aryl halides with amides, sulfonamides, and carbamates (Eq. (20)) [176]. Yin and Buchwald reported that palladium complexes containing Xantphos as a ligand gave good yields in a number of reactions between aryl bromides and these nucleophiles. The scope of the reaction remains narrower than that for amine substrates, but a variety of benzamides, acetamides, formamides, sulfonamides, and carbamates reacted with electron-poor aryl bromides to form good yields of the respective products. In addition, unactivated aryl bromides, iodides, and triflates reacted with benzamides, acetamides, N-methylformamide, and four-, five-, and six-membered ring lactams. Aryl chlorides, ortho-substituted aryl bromides, and N- alkyl acetamides were not suitable substrates. Moreover, the reactions were highly sensitive to temperature, as outlined in a footnote. Reactions performed at 100 C for 16 h led to full conversion, but those performed at 120 C, even with higher catalyst loadings, gave only low conversions. In addition, Edmundson reported the reaction of aryl halides with vinylogous amides using P,N ligand 13 (Eq. (21)) [177].
ð22Þ
Prior to the recent paper by Buchwald, an intermolecular version of the arylation of carbamates was published by Hartwig et al. (Eq. (22)) [163]. His group showed that reactions catalyzed by a combination of Pd(OAc)2 and P(tBu)3 formed N-aryl carbamates from aryl bromides or chlorides and tert-butyl carbamate, but that this system was inactive for reactions of amides or sulfonamides. Again, the reaction conditions were not as mild as those used for amination, but they were similar to those employed in the reactions with Xantphos. For the intermolecular reactions, the use of sodium phenoxide as base was crucial.
4.4.2
Allylamine as an Ammonia Surrogate
Allylamine can serve as an ammonia surrogate [178]. In the presence of palladium catalysts ligated by P(o-tolyl)3, diallylamine reacts with aryl bromides in modest yields. Reactions of allylamine with aryl and heteroaryl bromides catalyzed by (DPPF)PdCl2 give higher yields. Presumably, these reactions can be conducted in a more general fashion with the improved catalysts described above. Cleavage of the allyl group to give the parent arylamine was achieved using methanesulfonic acid and Pd/C.
1324 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
4.4.3
Imines
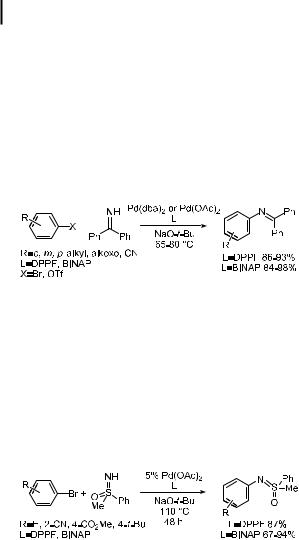
Benzophenone imine is commercially available and serves as an ammonia surrogate that reacts with aryl halides in high yields under standard palladium-catalyzed conditions. Catalysts based on both DPPFand BINAP-ligated palladium give essentially quantitative yields in reactions with aryl bromides (Eq. (23)). These reactions can be conducted with either Cs2CO3 or NaOtBu as the base [179, 180]. The products are readily isolated by chromatographic techniques or by crystallization. Alternatively, they can be cleaved to the parent aniline by addition of hydroxylamine, acid, or Pd/C [180].
ð23Þ
Sulfoximines have also proven to be suitable substrates for palladium-catalyzed CaN bond formation, as shown in Eq. (24), but the chemistry is less general than that with benzophenone imine [181]. Instead of serving as protected anilines, the products may be used as ligands for asymmetric catalysis. S-Methyl-S-phenyl sulfoximine was coupled with aryl bromides using P(o-tolyl)3, BINAP-, and DPPF-ligated palladium catalysts and alkoxide or carbonate as the base. Reaction times were long, but good yields were obtained in the case of electron-deficient aryl halides when either BINAP or DPPF was used as the ligand. Reactions of unactivated aryl halides gave good yields when an excess of the aryl halide was used or when the sulfoximine was added slowly. A full account of this work, including the synthesis of unsymmetrical, non-racemic, chiral N,N ligands, has recently appeared [182].
ð24Þ
4.4.4
Protected Hydrazines
Azoles can be produced from the products of palladium-catalyzed hydrazone arylation and can themselves serve as substrates for arylation reactions to produce N-aryl azoles by the Fischer indole synthesis. N-Aryl pyrazoles and related heterocycles can also be prepared after obtaining N-aryl hydrazines by palladium-catalyzed procedures. Benzophenone hydrazone was first found by both the Yale and MIT groups to be a particularly e ective substrate for palladium-catalyzed reactions, as summarized in Eq. (25) [183, 184]. Reactions of benzophenone hydrazone with either aryl bromides or iodides occur in high yields in the presence of either DPPFor BINAP-ligated palladium. These reactions are general and proceed with electron-rich, electron-poor, hindered, or unhindered aryl halides. The products of these re-
