
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
|
|
|
References |
103 |
|
|
|
|
|
13 |
M. Ishikura, M. Kamada, M. Terashima, |
|
J. J. Walsh, I. Tellitu, K. V. Bhaskar, |
|
|
Synthesis 1984, 936. |
|
T. R. Kelly, J. Org. Chem. 1998, 63, 1090. |
|
14 |
(a) M. B. Mitchell, P. J. Wallbank, |
32 |
T. R. Hoye, M. Chen, B. Hoang, L. Mi, |
|
|
Tetrahedron Lett. 1991, 32, 2273. (b) N. M. |
|
O. P. Priest, J. Org. Chem. 1999, 64, |
|
|
Ali, A. McKillop, M. B. Mitchell, R. A. |
|
7184. |
|
|
Rebelo, P. J. Wallbank, Tetrahedron 1992, |
33 |
C. B. de Koning, J. P. Michael, W. A. L. |
|
|
48, 8117. (c) N. W. Alcock, J. M. Brown, |
|
Otterlo, Tetrahedron Lett. 1999, 40, 3037. |
|
|
D. I. Hulmes, Tetrahedron: Asymmetry |
34 |
K. C. Nicolaou, J. M. Ramanjulu, S. |
|
|
1993, 4, 743. (d) D. Janietz, M. Bauer, |
|
Natarajan, S. Bra¨se, H. Li, C. N. C. |
|
|
Synthesis 1993, 33. (e) S. Achab, M. |
|
Boddy, F. Ru¨bsam, J. Chem. Soc., Chem. |
|
|
Guyot, P. Potier, Tetrahedron Lett. 1993, |
|
Commun. 1997, 1899. |
|
|
34, 2127. |
35 |
(a) K. C. Nicolaou, H. Li, C. N. C. |
|
15 |
W.-C. Shieh, J. A. Carlson, J. Org. Chem. |
|
Boddy, J. M. Ramanjulu, T.-Y. Tue, S. |
|
|
1992, 57, 379. |
|
Tatarajan, X.-J. Chu, S. Bra¨se, Chem. |
|
16 |
S. W. Wright, D. L. Hageman, L. D. |
|
Eur. J. 1999, 5, 2584. (b) K. C. Nicolaou, |
|
|
McClure, J. Org. Chem. 1994, 59, 6095. |
|
C. N. C. Boddy, H. Li, A. E. Koumbis, R. |
|
17 |
T. I. Wallow, B. M. Novak, J. Org. Chem. |
|
Hughes, S. Natarajan, N. F. Jain, J. M. |
|
|
1994, 59, 5034. |
|
Ramanjulu, S. Bra¨se, M. E. Solomon, |
|
18 |
G. Marck, A. Villinger, R. Buchecker, |
|
Chem. Eur. J. 1999, 5, 2602. (c) K. C. |
|
|
Tetrahedron Lett. 1994, 35, 3277. |
|
Nicolaou, A. E. Koumbis, M. |
|
19 |
T. Oh-e, N. Miyaura, A. Suzuki, J. Org. |
|
Takayanagi, S. Natarajan, N. F. Jain, T. |
|
|
Chem. 1993, 58, 2201. |
|
Bando, H. Li, R. Hughes, Chem. Eur. J. |
|
20 |
S. Yonezawa, T. Komurasaki, K. Kawada, |
|
1999, 5, 2622. |
|
|
T. Tsuri, M. Fuji, A. Kugimiya, N. Haga, |
36 |
D. Zhao, F. Xu, C. Chen, R. D. Tillyer, |
|
|
S. Mitsumori, M. Inagaki, T. Nakatani, |
|
E. J. J. Grabowski, P. J. Reider, C. Black, |
|
|
Y. Tamura, S. Takechi, T. Taishi, M. |
|
N. Ouimet, P. Prasit, Tetrahedron 1999, |
|
|
Ohtani, J. Org. Chem. 1998, 63, 5831. |
|
55, 6001. |
|
21 |
D. E. Zembower, H. Zhang, J. Org. |
37 |
V. N. Kalinin, O. S. Shilova, D. S. |
|
|
Chem. 1998, 63, 9300. |
|
Okladnoy, H. Schmidhammer, Mendeleev |
|
22 |
G. Lin, A. Zhang, Tetrahedron 2000, 56, |
|
Commun. 1996, 244. |
|
|
7163. |
38 |
S. Cerezo, M. Moreno-Manas, R. |
|
23 |
S. Kumar, J. Chem. Soc., Perkin Trans. 1 |
|
Pleixats, Tetrahedron 1998, 54, 7813. |
|
|
1998, 3157. |
39 |
M. Yoshida, K. Mori, Eur. J. Org. Chem. |
|
24 |
G. R. Geen, I. S. Mann, M. V. Mullane, |
|
2000, 1313. |
|
|
A. McKillop, Tetrahedron 1998, 54, |
40 |
S. R. Piettre, C. Andre, M.-C. Chanal, |
|
|
9875. |
|
J.-B. Ducep, B. Lesur, F. Piriou, P. |
|
25 |
D. L. Comins, G. M. Green, Tetrahedron |
|
Roboisson, J.-M. Rondeau, C. |
|
|
Lett. 1999, 40, 217. |
|
Schelcher, P. Zimmermann, A. J. |
|
26 |
(a) H. Koyama, T. Kamikaya, Tetrahedron |
|
Ganzhorn, J. Med. Chem. 1997, 40, 4208. |
|
|
Lett. 1997, 38, 3973. (b) H. Koyama, T. |
41 |
A. Ariffin, A. J. Blake, R. A. Ewin, W.-S. |
|
|
Kamikawa, J. Chem. Soc., Perkin Trans. 1 |
|
Li, N. S. Simpkins, J. Chem. Soc., Perkin |
|
|
1998, 203. |
|
Trans. 1 1999, 3177. |
|
27 |
J. Fu, V. Snieckus, Can. J. Chem. 2000, |
42 |
K. Kamikawa, T. Watanabe, A. Daimon, |
|
|
78, 905. |
|
M. Uemura, Tetrahedron 2000, 56, 2325. |
|
28 |
M. B. Goldfinger, K. B. Crawford, |
43 |
S. G. Nelson, M. A. Hilfiker, Org. Lett. |
|
|
T. M. Swager, J. Am. Chem. Soc. 1997, |
|
1999, 1, 1379. |
|
|
119, 4578. |
44 |
K. Kamikawa, M. Uemura, Synlett. 2000, |
|
29 |
M. B. Goldfinger, K. B. Crawford, |
|
938. |
|
|
T. M. Swager, J. Org. Chem. 1998, 63, |
45 |
C. Imrie, C. Loubser, P. Engelbrecht, |
|
|
1676. |
|
C. W. McCleland, J. Chem. Soc., Perkin |
|
30 |
F.-J. Zhang, C. Cortez, R. G. Harvey, J. |
|
Trans. 1 1999, 2513. |
|
|
Org. Chem. 2000, 65, 3952. |
46 |
Z. F. Plyta, D. Prim, J.-P. Tranchier, R. |
|
31 |
G. Bringmann, R. Go¨tz, P. A. Keller, R. |
|
Rose-Munch, E. Rose, Tetrahedron Lett. |
|
|
Walter, M. R. Boyd, F. Lang, A. Garcia, |
|
1999, 40, 6769. |
|

104 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
47 |
A. N. Cammidge, K. V. L. Crepy, Chem. |
72 |
A. J. Goodman, S. P. Stanforth, B. |
|
Commun. 2000, 1723. |
|
Tarbit, Tetrahedron 1999, 55, 15067. |
48 |
Y. Gong, H. W. Pauls, Synlett 2000, 829. |
73 |
B. U. W. Maes, G. L. F. Lemiere, R. |
49 |
C. N. Johnson, G. Stemp, N. Anand, S. |
|
Dommisse, K. Augustyne, A. Haemers, |
|
C. Stephen, T. Gallagher, Synlett 1998, |
|
Tetrahedron 2000, 56, 1777. |
|
1025. |
74 |
A. J. Cocuzza, D. R. Chidester, S. Culp, |
50 |
A. Fu¨rstner, J. Grabowski, C. W. |
|
L. Fitzgerald, P. Gilligan, Biorg. Med. |
|
Lehmann, J. Org. Chem. 1999, 64, 8275. |
|
Chem. Lett. 1999, 9, 1063. |
51 |
K. S. Chan, X. Zhou, M. T. Au, C. Y. |
75 |
S. Toyota, C. R. Woods, M. Benaglia, |
|
Tam, Tetrahedron 1995, 51, 3129. |
|
J. S. Siegel, Tetrahedron Lett. 1998, 39, |
52 |
Y. Deng, C. K. Chang, D. G. Nocera, |
|
2697. |
|
Angew. Chem. Int. Ed. 2000, 39, 1066. |
76 |
A. Mouaddib, B. Joseph, A. Hasnaoui, |
53 |
C. K. Chang, N. Bag, J. Org. Chem. 1995, |
|
J.-Y. Merour, Synthesis 2000, 549. |
|
60, 7030. |
77 |
M. Havelkova, M. Hocek, M. Cesnek, D. |
54 |
R. D’Alessio, A. Rossi, Synlett 1996, 513. |
|
Dvorak, Synlett 1999, 1145. |
55 |
J. Cossy, D. Belotti, Tetrahedron 1999, 55, |
78 |
T. Watanabe, N. Miyaura, A. Suzuki, |
|
5145. |
|
Synlett 1992, 207. |
56 |
(a) Q. Zheng, Y. Yang, A. R. Martin, |
79 |
(a) D. Muller, J.-P. Fleury, Tetrahedron |
|
Heterocycles 1994, 37, 1761. (b) I. |
|
Lett. 1991, 32, 2229. (b) Y. Fukuyama, Y. |
|
Kawasaki, M. Yamashita, S. Ohta, Chem. |
|
Kiriyama, M. Kodama, Tetrahedron Lett. |
|
Pharm. Bull. 1996, 44, 1831. |
|
1993, 34, 7637. |
57 |
B. Malapel-Andrieu, J.-Y. Merour, |
80 |
M. H. Abraham, P. L. Grellier, The |
|
Tetrahedron 1998, 54, 11079. |
|
Chemistry of the Metal–Carbon Bond (Eds.: |
58 |
C. B. de Koning, J. P. Michael, A. L. |
|
F. R. Hartley, S. Patai), J. Wiley, New |
|
Rousseau, Tetrahedron Lett. 1998, 39, 8725. |
|
York, 1985, Vol. 1, p. 115. |
59 |
C. B. de Koning, J. P. Michael, A. L. |
81 |
(a) P. Rocca, F. Marsais, A. Godard, G. |
|
Rousseau, J. Chem. Soc., Perkin Trans. 1 |
|
Queguiner, Tetrahedron Lett. 1993, 34, |
|
2000, 1705. |
|
2937. (b) F. Guillier, F. Nivoliers, A. |
60 |
Y. Nakano, Y. Yoshikawa, H. Kondo, J. |
|
Godard, F. Marsais, G. Queguiner, |
|
Am. Chem. Soc. 1986, 108, 7630. |
|
Tetrahedron Lett. 1994, 35, 6489. |
61 |
Y. Nakano, D. Imai, Synthesis 1997, 1425. |
82 |
T. R. Kelly, A. Garcia, F. Lang, J. J. |
62 |
S. Nakahara, J. Matsui, A. Kubo, |
|
Walsh, K. V. Bhaskar, M. R. Boyd, |
|
Tetrahedron Lett. 1998, 39, 5521. |
|
R. Go¨tz, P. A. Keller, R. Walter, G. |
63 |
H. Zhang, F. Xue, T. C. W. Mark, K. S. |
|
Bringmann, Tetrahedron Lett. 1994, 35, |
|
Chang, J. Org. Chem. 1996, 61, 8002. |
|
7621. |
64 |
K. Sheng-Chu, Y. L. Fang, T. Che-Ming, |
83 |
C. Griffiths, N. E. Leadbeater, |
|
Eur. Pat. Appl. 0667345 A1, 1995 (C.A.: |
|
Tetrahedron Lett. 2000, 41, 2487. |
|
123: 340113j). |
84 |
(a) H. Zhang, K. S. Chan, Tetrahedron |
65 |
V. Collot, P. Dallemagne, P. R. Bovy, S. |
|
Lett. 1996, 37, 1043. (b) H. Zhang, F. Y. |
|
Rault, Tetrahedron 1999, 55, 6917. |
|
Kwong, Y. Tian, K. S. Chan, J. Org. |
66 |
C. Enguehard, J.-L. Renou, V. Collot, |
|
Chem. 1998, 63, 6886. |
|
M. Hervet, S. Rault, A. Gueiffier, J. |
85 |
J. M. Saa, G. Martorell, J. Org. Chem. |
|
Org. Chem. 2000, 65, 6572. |
|
1993, 58, 1963. |
67 |
H. Nakamura, M. Aizawa, D. Takeuchi, |
86 |
E. M. Cempi, W. R. Jackson, S. M. |
|
A. Murai, O. Shimoura, Tetrahedron Lett. |
|
Maruccio, C. G. M. Naeslund, J. Chem. |
|
2000, 41, 2185. |
|
Soc., Chem. Commun. 1994, 2395. |
68 |
L. Revesz, F. Bonne, P. Makavou, |
87 |
A. M. Schoevaars, W. Kruizinga, |
|
Tetrahedron Lett. 1998, 39, 5171. |
|
R. W. J. Zijlstra, N. Veldman, A. L. Spek, |
69 |
C. Plisson, J. Chenault, Heterocycles |
|
B. L. Feringa, J. Org. Chem. 1997, 62, 4943. |
|
1999, 51, 2627. |
88 |
W. Shen, Synlett 2000, 737. |
70 |
S. M. Chi, J.-K. Choi, E. K. Yun, D. Y. |
89 |
W. Shen, L. Wang, J. Org. Chem. 1999, |
|
Chi, Tetrahedron Lett. 2000, 41, 919. |
|
64, 8873. |
71 |
I. Parrot, Y. Rival, C. G. Wermuth, |
90 |
W. R. Roush, M. L. Reilly, K. Kayama, |
|
Synthesis 1999, 1163. |
|
B. B. Brown, J. Org. Chem. 1997, 62, 8708. |
|
|
|
References |
105 |
|
|
|
|
|
91 |
M. Beller, H. Fischer, W. A. Herr- |
111 |
(a) T. Oh-e, N. Miyaura, A. Suzuki, |
|
|
¨ |
|
Synlett. 1990, 221. (b) T. Oh-e, N. |
|
|
mann, K. Ofele, C. Brossmer, Angew. |
|
||
|
Chem. Int. Ed. Engl. 1995, 34, 1848. |
|
Miyaura, A. Suzuki, J. Org. Chem. 1993, |
|
92 |
D. A. Alonso, C. Najera, Ma. C. |
|
58, 2201. |
|
|
Pacheco, Org. Lett. 2000, 2, 1823. |
112 |
Y. Hoshino, N. Miyaura, A. Suzuki, |
|
93 |
D. Zim, A. S. Gruber, G. Ebeling, J. |
|
Bull. Chem. Soc. Jpn. 1988, 61, 3008. |
|
|
Dupont, A. L. Monteiro, Org. Lett. 2000, |
113 |
S. Abe, N. Miyaura, A. Suzuki, Bull. |
|
|
2, 2881. |
|
Chem. Soc. Jpn. 1992, 65, 2863. |
|
94 |
A. Zapf, M. Beller, Chem. Eur. J. 2000, 6, |
114 |
Q. Zheng, Y. Yang, A. R. Martin, |
|
|
1830. |
|
Tetrahedron Lett. 1993, 34, 2235. |
|
95 |
M. Beller, J. G. E. Krauter, A. Zapf, |
115 |
C.-W. Lee, Y. J. Chung, Tetrahedron Lett. |
|
|
Angew. Chem. Int. Ed. Engl. 1997, 36, |
|
2000, 41, 3423. |
|
|
772. |
116 |
P. R. Eastwood, Tetrahedron Lett. 2000, 41, |
|
96 |
C. J. Mathews, P. J. Smith, T. Welton, |
|
3705. |
|
|
Chem. Commun. 2000, 1249. |
117 |
G. M. Boland, D. M. X. Dannelly, J.-P. |
|
97 |
T. Y. Zhang, M. J. Allen, Tetrahedron |
|
Finet, M. D. Rea, J. Chem. Soc., Perkin |
|
|
Lett. 1999, 40, 5813. |
|
Trans. I 1996, 2591. |
|
98 |
Deloxan THP II can be purchased from |
118 |
W. M. Clark, A. J. Kassick, M. A. |
|
|
Degussa AG. |
|
Plotkin, A. E. Eldridge, I. Lantos, Org. |
|
99 |
B. A. Lorsbach, J. T. Bagdanoff, R. B. |
|
Lett. 1999, 1, 1839. |
|
|
Miller, M. J. Kurth, J. Org. Chem. 1998, |
119 |
R. P. Robinson, E. R. Laird, J. F. Blake, |
|
|
63, 2244. |
|
J. Bordner, K. M. Donahue, L. L. |
|
100 |
K. H. Bleicher, J. R. Wareing, |
|
Lopresti-Morrow, P. G. Mitchell, M. R. |
|
|
Tetrahedron Lett. 1998, 39, 4587. |
|
Reese, L. M. Reeves, E. J. Stam, S. A. |
|
101 |
S. Wendeborn, S. Berteina, W. K.-D. |
|
Yocum, J. Med. Chem. 2000, 43, 2293. |
|
|
Brill, A. De Mesmaeker, Synlett. 1998, |
120 |
N. Yasuda, M. A. Huffman, G.-J. Ho, |
|
|
671. |
|
L. C. Xavier, C. Yang, K. M. Emerson, |
|
102 |
See ref. 101 and refs. cited therein. |
|
F.-R. Tsay, Y. Li, M. H. Kress, D. L. |
|
103 |
T. Ishiyama, M. Murata, N. Miyaura, J. |
|
Rieger, S. Karady, P. Sohar, N. L. |
|
|
Org. Chem. 1995, 60, 7508. |
|
Abramson, A. E. DeCamp, D. J. Mathre, |
|
104 |
S. R. Piettre, S. Baltzer, Tetrahedron Lett. |
|
A. W. Douglas, H.-H. Dolling, E. J. J. |
|
|
1997, 38, 1197. |
|
Grabowski, P. J. Reider, J. Org. Chem. |
|
105 |
(a) C. R. Strauss, R. W. Trainor, Aust. J. |
|
1998, 63, 5438. |
|
|
Chem. 1995, 48, 1665. (b) S. Caddick, |
121 |
R.-Q. Pan, X.-X. Liu, M.-Z. Deng, J. |
|
|
Tetrahedron 1995, 51, 10403. |
|
Fluorine Chem. 1999, 95, 167. |
|
106 |
(a) M. Larhed, G. Linderberg, A. |
122 |
G. A. DeBoos, J. J. Fullbrook, W. M. |
|
|
Hallberg, Tetrahedron Lett. 1996, 37, |
|
Owton, J. M. Percy, A. C. Thomas, |
|
|
8219. (b) M. Larhed, A. Hallberg, J. Org. |
|
Synlett 2000, 963. |
|
|
Chem. 1996, 61, 9582. |
123 |
V. Percec, J.-Y. Bae, D. H. Hill, J. Org. |
|
107 |
C. M. Huwe, H. Ku¨nzer, Tetrahedron Lett. |
|
Chem. 1995, 69, 1060. |
|
|
1999, 40, 683. |
124 |
(a) S. Gronowitz, A.-B. Ho¨rnfeldt, V. |
|
108 |
In addition to those references cited above, |
|
Kristjansson, T. Musil, Chem. Scripta |
|
|
see: (a) B. J. Backes, J. A. Ellam, J. Am. |
|
1986, 26, 305. (b) W. J. Thompson, J. H. |
|
|
Chem. Soc. 1994, 116, 11171. (b) B. |
|
Jones, P. A. Lyle, J. E. Thies, J. Org. |
|
|
Ruhland, A. Bombrun, M. A. Gallop, J. |
|
Chem. 1988, 53, 2052. (c) M. B. Mitchell, |
|
|
Org. Chem. 1997, 62, 7820. (c) R. |
|
P. J. Wallbank, Tetrahedron Lett. 1991, 32, |
|
|
Frenette, R. W. Friesen, Tetrahedron Lett. |
|
2273. (d) M. Uemura, H. Nishimura, K. |
|
|
1994, 35, 9177. (d) Y. Han, A. Giroux, C. |
|
Kamikawa, K. Nakayama, Y. Hayashi, |
|
|
Lepine, F. Laliberte, Z. Huang, H. |
|
Tetrahedron Lett. 1994, 35, 1909. S. Saito, |
|
|
Perrier, C. I. Bayly, R. N. Young, |
|
M. Sakai, N. Miyaura, Tetrahedron Lett. |
|
|
Tetrahedron 1999, 55, 11669. |
|
1996, 37, 2993. (g) W. Shen, Tetrahedron |
|
109 |
G. E. Atkinson, P. M. Fischer, W. C. |
|
Lett. 1997, 38, 5575. (h) W. A. Herrmann, |
|
|
Chan, J. Org. Chem. 2000, 65, 5048. |
|
C.-P. Reisinger, M. Spiegler, J. |
|
110 |
R. Franzen, Can. J. Chem. 2000, 78, 957. |
|
Organomet. Chem. 1998, 557, 93. (i) F. |
|

106 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
|
Firooznia, C. Gude, K. Chan, Y. Satoh, |
|
1999, 32, 1073. (b) J. Baur, M. F. Rubner, |
|
Tetrahedron Lett. 1998, 39, 3985. |
|
D. Boils, Macromolecules 1998, 31, 964. |
125 |
(a) V. V. Grushin, H. Alper, Chem. Rev. |
|
(c) W.-L. Yu, J. Pei, Y. Cao, W. Huang, |
|
1994, 94, 1047. (b) N. Miyaura, A. |
|
A. J. Heeger, Chem. Commun. 1999, 1837. |
|
Suzuki, Chem. Rev. 1995, 95, 2457. |
|
(d) M. Ranger, M. Leclerc, Can. J. Chem. |
126 |
(a) A. F. Littke, G. C. Fu, Angew. Chem. |
|
1998, 76, 1571. (e) A. Donat-Bouillud, |
|
Int. Ed. 1998, 37, 3387. (b) A. F. Littke, C. |
|
I. Levesque, Y. Tao, M. D’Iorio, S. |
|
Dai, G. C. Fu, J. Am. Chem. Soc. 2000, |
|
Beaupre, P. Blondin, M. Ranger, J. |
|
122, 4020. |
|
Bouchard, M. Leclerc, Chem. Mater. |
127 |
D. W. Old, J. P. Wolfe, S. L. Buchwald, |
|
2000, 12, 1931. (f ) D.-C. Shin, J.-H. Ahn, |
|
J. Am. Chem. Soc. 1998, 120, 9722. |
|
Y.-H. Kim, A.-K. Kwon, J. Polym. Sci. Part |
128 |
O. Lohse, P. Thevenin, E. Waldvogel, |
|
A 2000, 38, 3086. (g) S. Hotta, H. |
|
Synlett 1999, 45. |
|
Kimura, S. A. Lee, T. Tamaki, J. |
129 |
(a) J. P. Wolfe, R. A. Singer, B. H. Yang, |
|
Heterocycl. Chem. 2000, 37, 281. (h) A. |
|
S. L. Buchwald, J. Am. Chem. Soc. 1999, |
|
Izumi, M. Teraguchi, R. Nomura, T. |
|
121, 9550. (b) J. P. Wolfe, S. L. |
|
Masuda, Macromolecules 2000, 33, 5347. |
|
Buchwald, Angew. Chem. Int. Ed. Engl. |
138 |
M. Johannsen, K. A. Jorgensen, X.-F. |
|
1999, 38, 2413. |
|
Zheng, Q.-S. Hu, L. Pu, J. Org. Chem. |
130 |
Ligands 42 and 43 are now commercially |
|
1999, 64, 299. |
|
available from Strem Chemical Co. |
139 |
B. Karakaya, W. Claussen, K. Gessler, |
131 |
M. Rottla¨nder, P. Knochel, J. Org. |
|
W. Saenger, A. D. Schlu¨ter, J. Am. |
|
Chem. 1998, 63, 203. |
|
Chem. Soc. 1997, 119, 3296. |
132 |
S.-K. Kang, H.-W. Lee, S.-B. Jang, P.-S. |
140 |
(a) V. Hensel, A. D. Schlu¨ter, Eur. J. |
|
Ho, J. Org. Chem. 1996, 61, 4720. |
|
Org. Chem. 1999, 451. (b) V. Hensel, A. |
133 |
S.-K. Kang, H.-C. Ryu, H.-J. Son, Synlett |
|
D. Schlu¨ter, Chem. Eur. J. 1999, 421. |
|
1998, 771. |
141 |
A. Dondoni, C. Chiglione, A. Marra, |
134 |
G. W. Kabalka, R. M. Pagni, C. M. Hair, |
|
M. Scoponi, J. Org. Chem. 1998, 63, |
|
Org. Lett. 1999, 1, 1423. |
|
9535. |
135 |
S. F. Nielsen, D. Peters, O. Axelsson, |
142 |
H. Irngartinger, T. Escher, Tetrahedron |
|
Synth. Commun. 2000, 30, 3501. |
|
1999, 55, 10753. |
136 |
P. Galda, M. Rehahn, Synthesis, 1996, 614. |
143 |
S. Wang, W. J. Oldham Jr., R. A. |
137 |
(a) M. Remmers, B. Mu¨ller, K. Martin, |
|
Hudack Jr., G. C. Bazan, J. Am. Chem. |
|
H.-J. Ra¨der, W. Ko¨hler, Macromolecules |
|
Soc. 2000, 122, 5695. |

107
4
Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
John F. Hartwig
Abstract
The transition metal catalyzed synthesis of arylamines by the reaction of aryl halides or triflates with primary or secondary amines has become a valuable synthetic tool for many applications. This process forms monoalkyl or dialkyl anilines, mixed diarylamines or mixed triarylamines, as well as N-arylimines, carbamates, hydrazones, amides, and tosylamides. The mechanism of the process involves several new organometallic reactions. For example, the CaN bond is formed by reductive elimination of amine, and the metal amido complexes that undergo reductive elimination are formed in the catalytic cycle in some cases by NaH activation. Side products are formed by b-hydrogen elimination from amides, examples of which have recently been observed directly. An overview that covers the development of synthetic methods to form arylamines by this palladium-catalyzed chemistry is presented. In addition to the synthetic information, a description of the pertinent mechanistic data on the overall catalytic cycle, on each elementary reaction that comprises the catalytic cycle, and on competing side reactions is presented. The review covers manuscripts that appeared in press before June 1, 2001. This chapter is based on a review covering the literature up to September 1, 1999. However, roughly one-hundred papers on this topic have appeared since that time, requiring an updated review.
4.1
Introduction
4.1.1
Synthetic Considerations
Arylamines are commonplace. They are part of molecules with medicinally important properties, of molecules with structurally interesting properties, of materials with important electronic properties, and of transition metal complexes with catalytic activity. An aryl– nitrogen linkage is present in nitrogen heterocycles such as indoles [1, 2] and benzopyrazoles, conjugated polymers such as polyanilines [3–9], and readily oxidizable triarylamines used in electronic applications [10–13]. The ability of aryl halides and triflates to form arylamines allows a single group to be used as a synthetic intermediate in aromatic carbon–
Modern Arene Chemistry. Edited by Didier Astruc
Copyright 8 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30489-4

1084 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
carbon cross-coupling and amination reactions during structure–activity studies or library synthesis in drug development.
Despite the simplicity of the arylamine moiety, synthesis of these materials is often di - cult. Procedures involving nitration, reduction, and substitution are incompatible with many functional groups and often require protection and deprotection steps. Reductive amination provides a convenient route to some alkyl arylamines, but this procedure requires a preexisting aromatic CaN bond [14–17]. The addition of amines or alcohols to benzyne intermediates leads to variable regiochemistry [18, 19], and direct nucleophilic substitution of aryl halides typically requires a large excess of reagent, a highly polar solvent, and either high reaction temperatures or highly activated aryl halides [20, 21]. Alternatively, transition metal arene complexes have been used to accelerate substitution of the aryl halide. In this case, stoichiometric amounts of the transition metal complex are required [22, 23]. Thus, the new, mild, general catalytic method for the replacement of aryl halogen or sulfonate with amine provides an invaluable route to arylamines.
The procedure that is most competitive with the palladium chemistry is the traditional copper-mediated (Ullmann) substitution. Some recent progress has been made toward increasing the scope of these reactions and reducing the reaction temperatures [24, 25]. However, the reactions still typically require elevated temperatures [26–29], often precluding chemistry with sensitive functionalities. Further, these reactions often give products arising from diarylation of primary arylamine substrates. The Ullmann couplings are also substratespecific. Reactions of primary alkylamines and even dialkylamines give lower yields than do reactions of more acidic substrates such as arylamines, amides, and azoles. Reactions that form aromatic carbon–nitrogen bonds with weakly nucleophilic nitrogen substrates such as imidazoles and amides have been recently accomplished by a di erent copper-catalyzed process: coupling of arylboronic acids with the nitrogen nucleophile [30–39]. This chemistry is mild, but requires two steps from an aryl halide because of the intermediacy of the boronic acid.
Prior to the development of palladium-catalyzed amination chemistry, palladium-catalyzed coupling had been a powerful method of forming new CaC bonds starting from aryl halides or triflates [40–46]. A variety of main group and transition metal reagents have been used as the source of the carbon nucleophile. Tin and boron are most commonly used, but aluminum, zinc, magnesium, and silicon reagents are also e ective in this ‘‘cross-coupling’’ chemistry. Nickel and palladium complexes are now the most common catalysts. The crosscoupling chemistry has been reviewed a number of times, and several review articles are cited in the aforementioned references.
4.1.2
Prior CxX Bond-Forming Coupling Chemistry Related to the Amination of Aryl Halides
There is a substantial body of literature on the palladiumand nickel-catalyzed formation of aryl sulfides, selenides, and phosphines from aromatic and heteroaromatic halides. Progress on these reactions has continued with several recent contributions [47–50]. A review in 1997 covered the types of transformations that can be conducted and the types of catalysts used [51]. Particularly useful examples are the conversions of binaphthol to binaphthylphosphines

4.1 Introduction 109
via the triflate intermediate in the presence of palladium and nickel catalysts [52, 53]. The soft, nucleophilic character of thiolates and phosphides favors formation of the palladium thiolate or phosphide complexes and reductive elimination [54] of phosphine and sulfide.
4.1.3
Novel Organometallic Chemistry
Reductive elimination to form CaC and CaH bonds [55] generates the organic product in cross-coupling processes, as well as many other transition metal catalyzed reactions. Reductive elimination reactions comprise an early chapter in any organometallic text. Similarly, the cleavage of CaH bonds by oxidative addition, including the CaH bond in methane, is now known [56]. Even some remarkably mild CaC cleavage reactions have now been observed with soluble transition metal complexes [57–62].
In contrast, examples of complexes that undergo reductive elimination to form the CaN bond in amines have been uncovered only recently [63–68]. These reductive eliminations are the crucial CaN bond-forming steps of the aryl halide and triflate amination chemistry discussed in this review. Information on how these reactions occur and what types of complexes favor this process has been crucial to the understanding and development of new amination catalysts [64].
The cleavage of alkylamine NaH bonds by late transition metals to form metal amido complexes is also rare [69, 70]. When the transition metal is a low valent, late metal, the resulting amido complexes are highly reactive [71, 72]. It appears that the amination of aryl halides can involve an unusual NaH activation process by a palladium alkoxide to form a highly reactive palladium amide [65, 73].
In general, catalytic organometallic chemistry that forms carbon–heteroatom bonds is less developed than that which forms CaC bonds, although the Wacker process is classic catalytic organometallic chemistry [74, 75]. Other processes that form carbon–heteroatom bonds by homogeneous catalysis include oxidative carbonylations of amines and alcohols [75]. Nonoxidative carbonylation includes the reaction between an aryl halide, CO, and an alcohol or amine to form esters or amides [75], but these reactions may not involve alkoxo or amido intermediates. The amination and aquation of alkenes could form the CaX bonds in alcohols or amines, but an e cient, intermolecular aquation or hydroamination of alkenes is a highly sought-after process that remains rare [76]. Some interesting intramolecular examples [77– 79] and some slow intermolecular examples [80, 81] are known. Thus, the selectivities, deactivation mechanisms, and potential transformations of alkoxo and amido intermediates in catalytic chemistry are not well understood.
4.1.4
Organization of the Review
This review covers palladium-catalyzed amination of aryl halides and sulfonates. The nickelcatalyzed process [82–85] requires much higher catalyst loads and has a narrower substrate scope. Thus, it is not reviewed. Sections 4.2 to 4.5 cover the development of di erent palladium catalysts for the synthesis of arylamines and related structures. This work has
1104 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
stemmed from Kosugi’s initial finding [86, 87] that palladium complexes catalyze the formation of arylamines from tin amides and aryl halides. Section 4.6 covers the various arenas in which the palladium chemistry has been applied. Finally, Section 4.7 presents current mechanistic data concerning these processes with di erent catalysts.
4.2
Background
4.2.1
Early Palladium-Catalyzed Amination
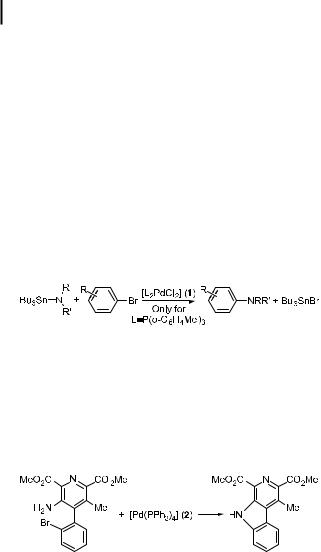
In the 1980s, a few results suggested that a general metal-catalyzed method of forming arylamines from aryl halides would be possible. In 1983, Kosugi, Kameyama, and Migita published a short paper on the reaction of tributyltin amides with aryl bromides catalyzed by [P(o-C6H4Me)3]2PdCl2 (1), as shown in Eq. (1) [86, 87].
ð1Þ
The scope of this reaction appeared to be limited to dialkylamides and electron-neutral aryl halides. For example, nitro-, acyl-, methoxy-, and dimethylamino-substituted aryl halides gave poor yields upon palladium-catalyzed reaction with tributyltin diethylamide. Further, aryl bromides were the only aryl halides to give any reaction product. Vinyl bromides gave modest yields of enamines in some cases. Only unhindered dialkyl tin amides gave substantial amounts of amination product. The mechanism did not appear to involve radicals or benzyne intermediates.
ð2Þ
Boger reported studies on palladium-mediated cyclization to form the CDE ring system of lavendamycin, as shown in Eq. (2) [88–90]. These reactions were conducted with stoichiometric amounts of [Pd(PPh3)4] (2). When used in a 1 mol % quantity, 2 failed to catalyze these reactions, presumably because of the absence of a base. Until almost ten years later, no palladium-catalyzed amination chemistry was reported, and there were few citations of the early amination chemistry.
In 1994, Paul, Patt, and Hartwig showed that the Pd(0) catalyst in Kosugi’s process was fPd[P(o-C6H4Me)3]2g (3), which underwent oxidative addition of aryl halides to give dimeric aryl halide complexes (4) [91]. These aryl halide complexes reacted directly with tin amides to form arylamine products (Eq. (3)). Thus, this chemistry could formally be viewed as being roughly parallel to Stille coupling.

4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 111
ð3Þ
In the same year, Guram and Buchwald showed that the use of in situ derived tin amides extended this chemistry beyond just electron-neutral aryl halides [92]. However, reactions that gave yields of 80 % or more were still limited to tin amides derived from secondary amines.
4.2.2
Initial Synthetic Problems to be Solved
The initial results concerning aryl halide amination and related results in chemistry forming aryl sulfides [93–96] and phosphines [97] strongly suggested that a mild, convenient route to arylamines from aryl halides could be developed. However, the source of the amido group had to be less toxic, more thermally stable, and less air-sensitive than a tin amide. The type of aryl halide amenable to this reaction had to be extended beyond electron-neutral aryl halides. Aryl chlorides and iodides, along with aryl triflates and less reactive, but more convenient, sulfonates needed to be included in the substrates capable of undergoing amination. Of course, heteroaromatic amines and halides are also important substrates and also needed to be included. Perhaps most importantly, reactions of primary amines needed to be developed. Finally, the rates and turnover numbers provided by the catalysts had to be much higher than those in the Kosugi and Migita chemistry and in Boger’s stoichiometric cyclization reaction. Faster rates would allow for the use of weaker bases and lower reaction temperatures. Over the past seven years, each of these goals has been achieved.
4.3
Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates
4.3.1
Early Work
4.3.1.1 Initial Intermolecular Tin-Free Aminations of Aryl Halides
In 1995, Hartwig and Buchwald published concurrently their respective groups’ results on tin-free amination of aryl halides [98, 99]. Instead of isolating or generating a tin amide in situ, the amination reactions were conducted by allowing an aryl halide to react with a combination of an amine and either an alkoxide or silylamide base (Eq. (5)).
ð5Þ
These reactions were typically conducted between 80 and 100 C in toluene solution. The catalysts used initially were 1, 3, or a combination of [Pd2(dba)3] (5a) (dba ¼ trans,trans-

1124 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
dibenzylidene acetone) and P(o-C6H4CH3)3. Catalysts used subsequently will be described below. Secondary amines were viable substrates, but primary amines gave substantial yields only with electron-poor aryl halides.
4.3.1.2 Initial Intramolecular Amination of Aryl Halides
Intramolecular aryl halide aminations were also conducted with the original catalysts [99]. For example, the reactions in Eq. (6) proceeded with yields in excess of 80 %. In this case, the halide could be iodide or bromide, and [Pd(PPh3)4] proved to be a more e ective catalyst than fPd[P(o-C6H4Me)3]2Cl2g.
ð6Þ
Buchwald later provided an extensive account of the intramolecular amination reactions [100]. Catalysts and conditions were optimized using triaryl and chelating phosphines, but the catalysts discussed in Section 4.4, developed after this work was published, would most probably provide milder conditions for these reactions. Screening of a variety of combinations of phosphine ligands and palladium precursors showed that chelating ligands such as Ph2P(CH2)nPPh2 (n ¼ 2–4) or 1,10-bis(diphenylphosphino)ferrocene (DPPF) gave good yields of cyclized product, as did a combination of Pd2(dba)3 and P(2-furyl)3, but none were better than [Pd(PPh3)4].
4.3.2
Second Generation Catalysts: Aryl Bis-phosphines
4.3.2.1 Amination of Aryl Halides
With the exception of the intramolecular amination reactions, all of the chemistry described above involved reactions catalyzed by palladium complexes containing the sterically hindered P(o-C6H4Me)3. Mechanistic studies, as described below, showed that the catalytic cycle involved exclusively mono-phosphine intermediates. However, stoichiometric studies on reductive elimination from PPh3-ligated palladium amides [63, 101] and on b-hydrogen elimination from related d8 square-planar iridium amides [102] suggested that palladium complexes with chelating ligands would be particularly e ective catalysts for the amination chemistry. In fact, many of the reasons why such complexes should be e ective for this amination process parallel the reasons why they are e ective for cross-coupling of aryl halides with main group alkyl reagents [103].
In papers published back-to-back in 1996, Hartwig and Buchwald reported amination chemistry with palladium complexes of DPPF and BINAP as catalysts [64, 104]. These palladium complexes facilitated aminations of aryl bromides and iodides with primary alkyl amines, with cyclic secondary amines, and with anilines. It is ironic that the amination chemistry was first discovered by using a particularly labile phosphine, but was dramatically improved by the use of tightly bound chelating ligands.
