
Astruc D. - Modern arene chemistry (2002)(en)
.pdf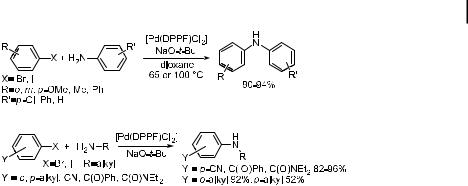
4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 113
ð7Þ
ð8Þ
DPPF-ligated palladium provided nearly quantitative yields for the amination of aryl halides with anilines (Eq. (7)). Electron-rich, electron-poor, hindered or unhindered aryl bromides or iodides all participated in the amination chemistry with only a few exceptions. Nitro haloarenes gave no amination product with aniline substrates. Aryl halides with carbonyl groups bearing enolizable hydrogens gave poor yields, and esters were converted to the tert-butyl ester by the tert-butoxide base. These groups have since been shown to be amenable to amination processes with Cs2CO3 as the base [105]. DPPF-ligated palladium also gave good yields of mixed alkyl arylamines with a variety of substrates (Eq. (8)). With electron-poor aryl halides, excellent yields of N-alkyl anilines were obtained. With electron-neutral aryl halides, 60–92 % yields were obtained, depending on the positions of the alkyl substituents [64, 106]. In the cases of unhindered and electron-neutral aryl halides coupled with unhindered primary amines, diarylation can occur. In these cases, excess amine suppressed the formation of diarylation products [107]. For most of these reactions, 5 mol % catalyst was employed, although 1 mol % can be used in most cases.
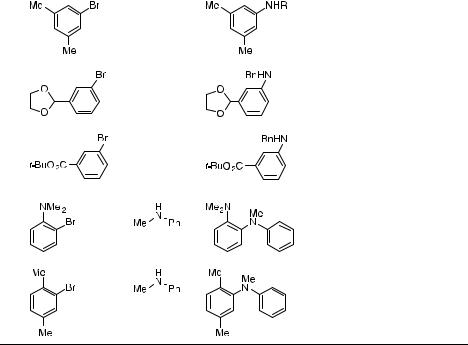
A full account of the palladium-catalyzed amination reactions involving BINAP complexes was published in 2000 [108]. BINAP-ligated palladium provides higher yields than DPPF in the case of electron-neutral aryl halides and primary alkyl amines, as shown in Table 1. The increased yields result largely from the lack of diarylation products. Slightly less reduction product is also observed, although the amount of arene formed when using BINAP or DPPF is low in both cases. Further, in favorable cases, it was shown that 0.05 mol % of the BINAPligated catalyst may be used. Racemic and resolved BINAP give identical results. Again, aryl iodides are suitable substrates. A subtle but sometimes important advantage of the chelating ligands is their ability to prevent racemization of amines that possess a stereogenic center a to nitrogen. Reactions of optically active amines have been evaluated; the racemization that sometimes occurs with the original P(o-tolyl)3 catalyst system does not occur when racBINAP is used as a ligand [109].
In general, BINAP-ligated palladium is exquisitely selective for the monoarylation of primary amines and is the preferred catalyst for reactions of primarily alkylamine substrates. This result is mutually exclusive with mild, high-yielding reactions of secondary amines with aryl halides to form tertiary amines. Thus, reactions of secondary amines with aryl halides catalyzed by BINAP-ligated palladium are less reliable. For example, reactions of morpholine gave good yields, but those of piperidine and of acyclic secondary amines gave low to modest yields. Exceptions to this rule include reactions of N-methyl aniline, of ortho-substituted aryl bromides, and of electron-poor aryl bromides. In fact, a particularly favorable case is the re-
114 |
4 |
Palladium-Catalyzed Amination of Aryl Halides and Sulfonates |
|
|
|
|||
|
Tab. 4.1. Selected aryl bromide aminations catalyzed by BINAP/Pd2(dba)3 |
|
|
|||||
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
Halide |
Amine |
Product |
Cat. (%) |
t (h) |
Isolated |
|
|
|
|
|
|
|
|
Yield (%) |
|
|
|
|
|
|
|
|
|
1 |
|
RNH2 |
|
R ¼ hexyl |
|
|
||
|
|
|
|
|
|
0.5 |
2 |
88 |
|
|
|
|
|
|
R ¼ Bn |
4 |
79 |
|
|
|
|
|
|
0.5 |
7 |
79 |
|
|
|
|
|
|
0.05 |
|
|
2 |
|
H2NBn |
|
0.5 |
2 |
81 |
||
3 |
H2NBn |
0.5 |
3.5 |
71 |
4 |
1.0 |
39 |
66 |
5 |
0.5 |
36 |
94 |
action of 2-bromo-p-xylene with N-methyl piperazine, which requires only 0.05 mol % catalyst. Reactions of primary and secondary amines with electron-poor aryl halides were also conducted with weak bases, as outlined in Section 4.5.
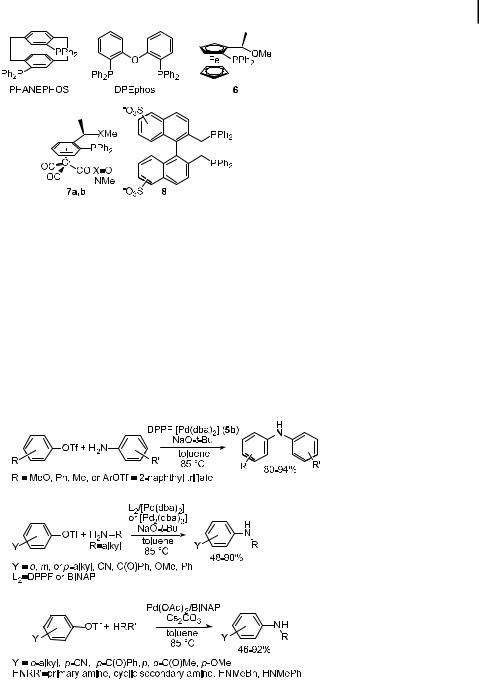
Related bis(phosphine) ligands and some additional aryl bis(phosphine)s and hemilabile arylphosphines have been used in the amination chemistry and may be valuable for certain aryl halide aminations (Figure 1). For example, PHANEPHOS has been shown to give good yields in the specific case of the resolution of 2,20-dibromo[2,2]paracyclophane [110]. More extensive studies have not been reported. Buchwald has reported that reactions with DPEphos [bis(2,20-diphenylphosphino)diphenyl ether] gave higher yields in the formation of certain diarylamines than did reactions with BINAP or DPPF as ligand [111]. As described in more detail below, solutions to the problem of coupling acyclic dialkylamines and the employment of milder bases began with the use of Kumada’s phosphino ether ligand 6 [105, 112]. In addition, Uemura has reported the amination of aryl bromides in the presence of arene-chromium complexes 7a,b as phosphino ether and phosphino amine analogues of Kumada’s ferrocene-based ligands. Good yields were observed for reactions of cyclic and acyclic secondary amines [113]. Finally, Boche has used a sulfonated BISBI ligand 8 to conduct aminations in a two-phase aqueous solution containing water/methanol, water/methanol/
4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 115
Fig. 1. Chelating and hemilabile ligands used in palladium-catalyzed amination of aryl halides.
toluene, or water/2-butanol. Electron-poor aryl halides reacted with aniline under these aqueous palladium-catalyzed conditions to give diarylamines [114].
In general, the fastest rates for the amination of aryl bromides with bis(phosphine) ligands are seen when sodium tert-butoxide is used as the base. However, Prashad has recently reported that bases containing b-hydrogens may be used, and, in some cases, reactions with sodium methoxide as the base occurred in higher yields than those with sodium tertbutoxide [115]. The improvement in reaction yield was first observed for a coupling of benzophenone imine with a pendant carbamate. However, this study showed high yields for reactions of primary and secondary amines, including anilines, when using BINAP as the ligand and methoxide or isopropoxide as the base.
ð9Þ
ð10Þ
ð11Þ
4.3.2.2 Amination of Aryl Triflates
Palladium(0) complexes containing P(o-C6H4Me)3 as a ligand show low reactivity toward aryl triflates [116, 117]. Thus, the original catalyst is not e ective for the amination of aryl tri-

1164 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
flates. However, palladium complexes with the chelating phosphines DPPF and BINAP are e ective [118, 119]. Selected aminations of aryl triflates by aniline are shown in Eq. (9), and selected aminations of aryl triflates by alkylamines are shown in Eq. (10). As in the case of the amination of aryl halides, DPPF is an e ective ligand for amination reactions involving anilines or aminations involving electron-poor aryl triflates. The yields for formation of mixed diarylamines have exceeded 90 % in all the examples explored [118]. Reactions of electron-neutral aryl halides with alkylamines gave yields in the range 42–75 % when DPPF was the ligand. This combination of substrates gave yields in the range 54–77 % when BINAP or Tol-BINAP was the ligand; several examples, particularly the triflate derived from the p- OMe-substituted phenol, showed higher yields in reactions conducted with BINAP as the ligand than in those conducted with DPPF.
The reactions of electron-poor aryl triflates were plagued by triflate cleavage to form phenol, presumably due to the stable phenolate that initially results from cleavage. In some cases, this problem could be overcome by slow addition of the triflate, whereby high-yielding amination could be achieved [118]. The problem of triflate cleavage was reduced in a more general fashion by using Cs2CO3 as the base, as shown in Eq. (11) [120]. Under these conditions, primary and secondary amines reacted in high yields with both electron-poor and electron-rich aryl triflates in the presence of BINAP-ligated palladium as the catalyst. However, no reactions of unhindered, unactivated aryl triflates with primary amines were reported with Cs2CO3 as the base, and this combination gave modest yields of 47–65 % when NaOtBu was the base. Reactions performed in toluene solution gave higher yields than those performed in THF, although good yields were obtained in THF in some cases.
Recently, Torisawa reported that the addition of 18-crown-6 promotes the reactions of aryl triflates when Cs2CO3 is used as the base [121]. This study focused on the reactions of N-Boc piperazine. In the absence of the crown ether, bis-N-Boc piperazine was produced as a byproduct, and reaction times were long. Yields for the reaction of 3,4-dichlorophenyl triflate with N-Boc piperazine were 35 % and 65 %, respectively, in the absence and presence of the crown ether. A similar e ect was also observed for the reaction of the analogous dichloro bromoarene.
4.3.2.3 Amination of Heteroaromatic Halides
Many nitrogen heterocycles bind strongly to late transition metals. As a result, heteroaromatic halides with basic nitrogens can displace weakly binding ligands such as P(o- C6H4Me)3. The original catalyst system containing P(o-C6H4Me)3 as ligand was consequently ine ective for aminations of heteroaromatic substrates that could bind to palladium. Indeed, pyridine displaces P(o-C6H4Me)3 from isolated palladium complexes to form pyridine-ligated species [122]. Chelating phosphines are less readily displaced by pyridines. Thus, the advent of amination reactions conducted with chelating ligands allowed the amination of pyridyl halides [123]. However, studies discussed below on third-generation catalysts show that chelating ligands are not required for the amination of pyridines.
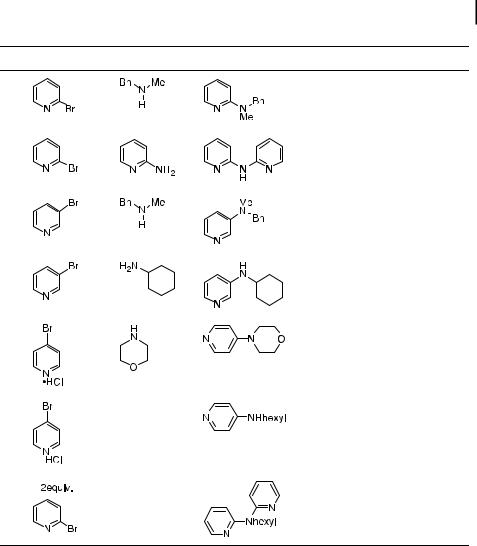
Results on the amination of pyridyl halides conducted with two phosphine ligands and two di erent palladium precursors have been published (Table 2) [123]. The most general palladium precursor proved to be palladium acetate. Again, BINAP was found to be generally e ective for amination with either primary or secondary amines. However, yields were lower with unbranched primary amines than those with branched amines, such as cyclo-
|
4.3 |
Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 117 |
|||||
Tab. 4.2. Palladium-catalyzed amination of pyridyl halides. |
|
|
|
|
|
||
halopyridine |
Amine |
product |
catalyst |
|
|
yield (%) |
|
1 |
|
|
Pd2(dba)3/DPPP |
86 |
|||
2 |
|
|
Pd2(dba)3 |
/DPPP |
87 |
||
3 |
|
|
Pd2(dba)3 |
/(G)BINAP |
77 |
||
4 |
|
|
Pd2(dba)3 |
/(G)BINAP |
82 |
||
5 |
|
|
Pd3 |
(OAc)6 |
/DPPP |
91 |
|
6 |
H2Nhexyl |
|
Pd3 |
(OAc)6 |
/(G)BINAP |
67 |
|
7 |
H2Nhexyl |
|
Pd3 |
(OAc)6 |
/(G)BINAP |
71 |
|
hexylamine. DPPP [bis(diphenylphosphine)propane], which is less expensive than BINAP, in combination with either 5a or Pd(OAc)2 (9), acted as an e ective catalyst system for the amination of pyridyl bromides by secondary amines or amines lacking hydrogens a to the nitrogen. As was the case for aminations of aryl halides conducted with DPPF and BINAP as ligand, acyclic secondary amines gave only low yields of the amino pyridines.
Hydrazinopyridines are important in the preparation of triazines and as intermediates in the production of agrochemicals and pharmaceuticals. Thus, Arterburn et al. investigated the reaction between hydrazine derivatives and pyridyl halides and triflates [124]. These workers found that 2-bromo- and 2-chloropyridine reacted with benzophenone hydrazone
1184 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
(see Section 4.4.5 for the use of hydrazones) to give good yields of the coupled product. However, pyridines with ester functionalities in the 5-position gave lower yields unless the pyridyl triflate was used. These reactions were conducted with BINAP as the ligand and NaOtBu as the base. In addition, these authors investigated the reactions of tert-butyl carbazate (tert-butylOC(O)NHNH2) with 2-bromopyridine. Using DPPF as the ligand and Cs2CO3 as the base, they obtained a 45 % yield of the pyridylhydrazine after acidic workup. Reactions of 2-bromo- and 2-chloropyridine with di-tert-butyl hydrazodiformate (tBuOC(O)NHNHC(O)OtBu) gave the coupled product in 45–85 % yield when this ligand and base were used. These reactions are surprising if one considers the potential for reduction of palladium by hydrazines. Pd(PPh3)4 is generally prepared using Pd(II) precursors and hydrazine as a reducing agent [125].
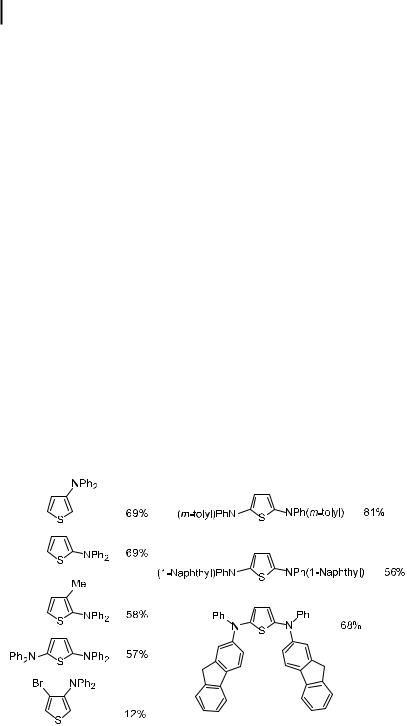
Aminations of five-membered heterocyclic halides, such as furans and thiophenes, are limited. These substrates are particularly electron-rich. As a result, oxidative addition of the heteroaryl halide and reductive elimination of the amine are slower than for simple aryl halides (see Sections 4.7.1 and 4.7.3). In addition, the amine products can be air-sensitive and require special conditions for their isolation. Nevertheless, Watanabe has reported examples of successful couplings between diarylamines and bromothiophenes [126]. Triarylamines are important for materials applications because of their redox properties, and these particular triarylamines should be especially susceptible to electrochemical oxidation. Chart 1 shows the products formed from the amination of bromothiophenes and the associated yields. As can be seen, 3-bromothiophene reacted in higher yields than 2-bromothiophene, but the yields were more variable with substituted bromothiophenes. In some cases, acceptable yields for double additions to dibromothiophenes were achieved. These reactions all employed a third-generation catalyst (vide infra), containing a combination of Pd(OAc)2 and P(tBu)3. The yields for reactions of these substrates were much higher in the presence of this catalyst than they were in the presence of arylphosphine ligands.
Chart 1

4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 119
4.3.2.4 Aminations of Solid-Supported Aryl Halides
Two groups [127, 128] have reported results on the solid-phase amination of aryl halides using both P(o-C6H4Me)3 and chelating ligands. It has been shown that Stille and Suzuki reactions are reliable, high-yielding processes for substrates loaded on solid supports [129]. Thus, supported aryl halides can now be used to form new CaC and CaN bonds, and presumably CaS, CaP, and CaO bonds as well.
Both Farina and Ward [127] at Boehringer Ingelheim and Willoughby and Chapman [128] at Merck have reported successful amination reactions of aryl halides supported on polystyrene Rink and Rapp TentaGel S RAM resin. The results closely parallel those obtained in the solution phase. Secondary amines were successfully coupled with solid-supported aryl halides in high yields with P(o-C6H4Me)3 as the ligand on palladium. Primary amines required either BINAP or DPPF for successful coupling with the aryl halides, and similar results were obtained with either ligand. NaH groups for which no arylation chemistry had been reported in the solution phase were likewise unreactive on the solid supports. For example, nitroanilines, aminotriazine, 5-aminouracil, 6-diaminoanthraquinone, histidine, 2- aminobenzimidazole, imidazole, and pyrazole gave no products of CaN bond formation in attempted solid-phase reactions in the presence of P(o-C6H4Me)3, DPPF, or BINAP-ligated palladium.
4.3.2.5 Amination of Polyhalogenated Aromatic Substrates
Multiple arylations of polybromobenzenes have been conducted to generate electron-rich arylamines. Tris(4-bromophenyl)amine and 1,3,5-tribromobenzene both react cleanly with N- aryl piperazines in the presence of either P(o-tolyl)3 or BINAP-ligated catalysts to form hexaamine products [130]. Reactions of other polyhalogenated arenes have also been reported with DPPF as the ligand [131, 132]. Competition between aryl bromides and iodides or aryl bromides and chlorides has been investigated in relation to the formation of aryl ethers [133], and similar selectivity should presumably be observed for the amination. In this case, bromochloroarenes reacted preferentially at the aryl bromide position. Sowa showed complete selectivity for amination of aryl-chloro, -bromo, or -iodo linkages over aryl-fluoro linkages [134]. This selectivity contrasts the faster fluoride substitution in uncatalyzed aromatic substitutions.
4.3.3
Third-Generation Catalysts with Alkylmonophosphines
Until recently, alkylphosphines had been used less often than arylphosphines in crosscoupling chemistry. However, several studies pointed to the potential of such ligands in the palladium-catalyzed amination of aryl halides. Alkylphosphines in combination with palladium catalyst precursors have now been shown to allow milder conditions for the amination of aryl bromides, to improve yields with acyclic secondary amines, to give high turnover numbers, and to induce mild aminations of inexpensive aryl chlorides and tosylates.
As discussed in more detail in Section 4.7, which reviews studies on the reaction mechanism, the major product that competes with the arylamine is the arene resulting from hydrodehalogenation of the aryl halide. Hartwig showed that steric hindrance was crucial to minimize formation of this side product when monophosphines are used [135]. A second side product from reaction of a primary amine is diaryl alkyl tertiary amine, resulting from

1204 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
arylation of the secondary amine product. Buchwald showed that chelating ligands such as BINAP minimize the formation of this product, presumably because of the increased steric demand at the metal center created by the four-coordinate amido intermediate containing a bis(phosphine) [104].
4.3.3.1 High-Temperature Aminations Conducted with P(tBu)3 as Ligand
Subsequent to these mechanistic and synthetic findings, Yamamoto, Nishiyama, and Koie at Tosoh Corporation reported high yield formation of aryl piperazines with turnover numbers as high as 7000 when P(tBu)3 was used as a ligand for palladium at high temperatures [136]. They found that unprotected piperazine reacted with aryl bromides at 120 C to form good yields of the monoarylated piperazines, which are useful pharmaceutical intermediates. The high turnover numbers demonstrate that the amination chemistry may be industrially feasible on a large scale for even the less expensive of speciality chemicals. In a subsequent paper, these authors showed that triarylamines, which are useful as components of lightemitting diodes, can be prepared from aryl halides and diamines with the same catalyst system with turnover numbers of 4000 [137]. These studies suggested that sterically hindered alkylphosphines can generate highly active catalysts for cross-coupling reactions.
4.3.3.2 Use of Sterically Hindered Bis(phosphine) Ligands
Amination of aryl bromides and chlorides: Hartwig first reported the high yielding amination of unactivated aryl chlorides under mild conditions by using DtBPF bis(di-tert-butyl- phosphino)ferrocene [138, 139] and similar sterically hindered bis(phosphine) ligands developed by Spindler, Togni, and Bla¨ser for asymmetric hydrogenations [140–142]. Activated aryl chlorides react much like unactivated aryl bromides, and reactions of these substrates with the original catalyst based on tri-ortho-tolyl phosphine were reported [143]. Nickel complexes are also known to react readily with aryl chlorides in cross-coupling chemistry [144–149], and they have also been used in the catalyzed amination of aryl chlorides [82, 83]. In general, the nickel-catalyzed chemistry occurs with lower turnover numbers and has a narrower substrate scope than the palladium-catalyzed reactions with third-generation ligands. For example, primary alkylamines are not suitable substrates for unhindered aryl halides when the nickel catalysts are used, although cyclic secondary amines and anilines can give good yields. Thus, the palladium, rather than nickel, chemistry will be described in detail.
ð12Þ
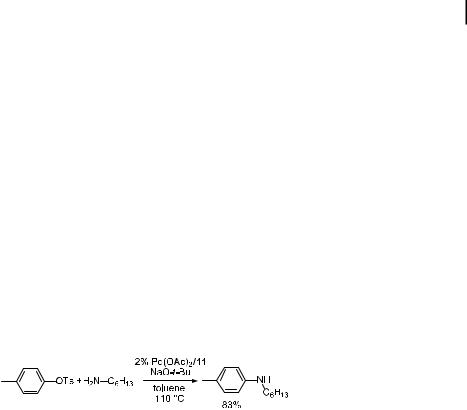
4.3 Palladium-Catalyzed Amination of Aryl Halides with Amine Substrates 121
Motivated by the mechanistic and synthetic studies with hindered monophosphines described in the previous paragraph and by the benefits of chelating ligands in controlling the selectivity in reactions with primary amines, Hartwig and Hamann employed the three sterically hindered alkyl bis(phosphine) ligands in Eq. (12) for the amination chemistry [150]. Reactions conducted with DtBPF (10) as the ligand gave high yields of amination products under mild conditions (80–100 C) with unactivated aryl halides and either anilines or cyclic secondary amines. Reactions of aryl bromides were remarkably fast and, for the first time, could be carried out at room temperature. However, this ligand did not give the anticipated high yields in reactions with primary amines. This result and additional results on the use of this ligand in ketone arylation and in the formation of diaryl ethers from aryl halides suggest that DtBPF acts as a monodentate ligand during the catalytic cycle.
Thus, commercially available sterically hindered bis(phosphine)s were tested for their selectivity with primary amines. The ligands 11 and 12 in Eq. (12) [142] are more constrained to a geometry that ensures chelation and may improve selectivity. Indeed, reactions of primary amines with unactivated aryl chlorides gave high yields of the coupled product with high selectivity for CaN coupling over hydrodehalogenation and for monoarylation of the primary amine. These ligands generate catalysts that provide the most favorable combination of monoarylation over diarylation, amination over hydrodehalogenation, and mild activation of aryl chlorides.
ð13Þ
Amination of aryl tosylates: Aryl tosylates are, of course, more convenient to prepare and are more stable reagents than aryl triflates, and they are generated from phenols using a cheaper sulfonating agent. However, the palladium-catalyzed coupling of aryl tosylates is rare. Only one paper has contained palladium-catalyzed coupling with arene sulfonates, and this work involved an electron-deficient aryl fluoroarene sulfonate [151]. One report of the carbonylation of an activated aryl tosylate has appeared [152]. Thus, it was remarkable that the complexes derived from ligand 11 were found to catalyze the amination of aryl tosylates as shown in Eq. (13). These reactions required 110 C for unactivated aryl tosylates, but the yields were high. Because of the tight chelation of these ligands, high selectivity is observed for reactions of primary amines with the unhindered and unactivated p-tolyl tosylate.
4.3.3.3 P,N Ligands and Dialkylphosphinobiaryl Ligands
Buchwald’s group showed that phosphino ether ligands developed by Kumada and Hayashi for asymmetric cross-coupling [153, 154] gave high yields in the formation of tertiary amines from aryl bromides and acyclic secondary amines [112]. Kocovsky showed that complexes of MAP, an amino analogue of MOP that is based on a binaphthyl structure, gives fast rates in the arylation of anilines [155]. To convert palladium complexes of these ligands into catalysts suitable for amination of aryl chlorides, Buchwald’s group prepared cyclohexyl analogues of MAP and of BINAP, one of which (ligand 13) is shown in Eq. (14) [156]. These ligands generate catalysts for high-yielding aminations of unhindered aryl bromides with secondary
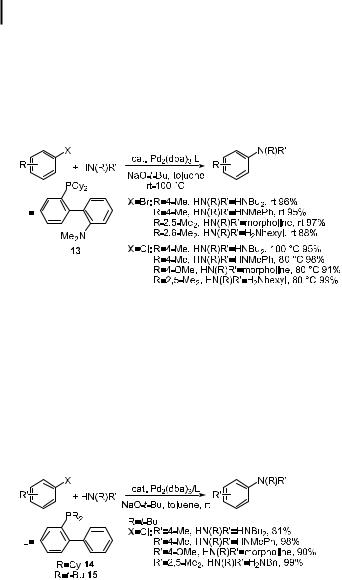
1224 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
amines and of ortho-substituted aryl bromides with primary amines at room temperature. The catalysts bearing these ligands also allow the amination of activated aryl chlorides at room temperature and of unactivated aryl chlorides at 80–100 C. The cyclohexyl MAP analogue 13 is generally more suitable for reactions of secondary amines than primary amines, but it does permit mild coupling chemistry of primary amines and ortho-substituted aryl halides.
ð14Þ
Although the P,O and P,N structures and their potential hemilability were part of the design and selection of these ligands [157], the nitrogen substituent proved to detract from rather than enhance the catalytic performance of palladium complexes of these ligands. Thus, their desamino analogues, dialkylphosphino-2-biphenyl ligands 14 and 15 in Eq. (15) generated more active catalysts and are simpler to prepare [156]. The dicyclohexylphosphino-2-biphenyl and di-tert-butylphosphino-2-biphenyl ligands allow room temperature amination of aryl chlorides in selected cases. For example, reactions that required elevated temperatures when the cyclohexylphosphino P,N ligand was used could be performed at room temperature with the 2-di-tert-butylphosphinobiphenyl ligand.
ð15Þ
A full account of the scope and limitations of the amination chemistry of these ligands has recently appeared [158]. With ligand 15, a number of aminations were conducted at room temperature. In the absence of an ortho substituent, couplings of primary amines with unactivated aryl chlorides at room temperature required 5 mol % catalyst. However, a variety of secondary amines, both cyclic and acyclic, reacted with activated or deactivated aryl chlorides at room temperature. Thirteen examples were demonstrated. The scope of this process was broader, however, when reactions were conducted at 80 or 110 C. Under these conditions, unactivated aryl chlorides reacted with a variety of amines in high yields. In favorable cases, such as reactions of aryl chlorides bearing one ortho methyl group, reactions of N-methyl
