
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
4.4 Aromatic C--N Bond Formation with Non-Amine Substrates and Ammonia Surrogates 133
actions can be converted to hydrazones that bear enolizable hydrogens and that are suitable for indole synthesis in the presence of an acid and a ketone [183].
Most recently, Wagaw, Yang, and Buchwald published a full account of the synthesis of indoles using the palladium-catalyzed amination process [185]. From the standpoint of catalysis, new results included improved turnover numbers and rates when Xantphos was used as ligand. Moreover, this ligand allowed diarylation of the hydrazone, including a onepot sequential diarylation to provide mixed diaryl hydrazones. A procedure for the alkylation of N-aryl hydrazones was also reported. These procedures allow the formation of N-aryl and N-alkyl indoles after subjecting the products to Fischer conditions for indole synthesis.
ð25Þ
An addition to the literature on the arylation of protected hydrazines was recently contributed by Skerlj et al. [186]. In addition to the reactions of halopyridines with tert-butyl carbazate (H2NNHBoc) discussed in Section 4.3.2.3, these authors have reported the reaction of electron-deficient aryl bromides with this substrate. Reactions occurred when using DPPF or the ferrocenyl 11 as the ligand along with Cs2CO3 as the base at temperatures of 100–110 C.
4.4.5
Azoles
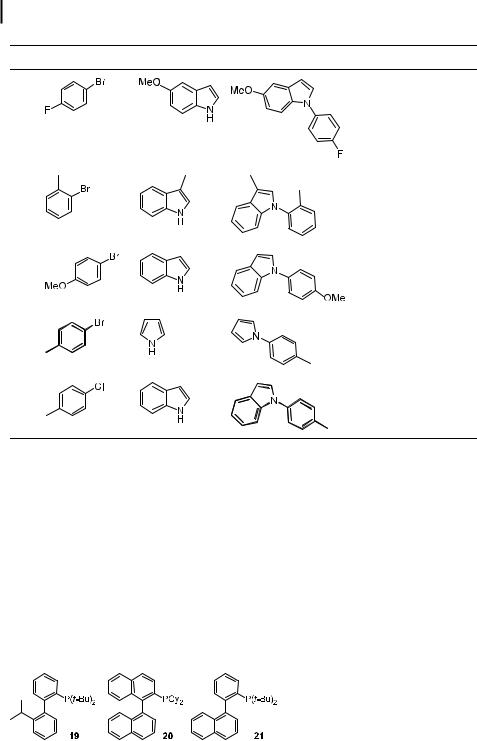
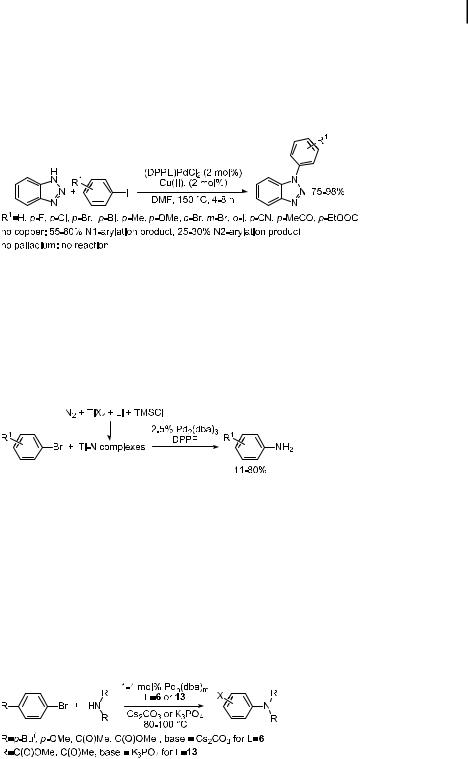
Indoles, pyrroles, and carbazoles are themselves suitable substrates for palladium-catalyzed amination. An initial study of this reaction using DPPF-ligated palladium as the catalyst showed that these reactions occurred readily with electron-poor aryl halides. With unactivated aryl bromides, reactions with pyrrole or indole resulted in good yields, but reaction times were long and a temperature of 120 C was required. Thus, an improved catalyst system was necessary to make the reaction more general and amenable to temperatureor basesensitive substrates.
Sterically hindered alkylmonophosphines provided improved catalyst systems (Table 5) [163]. In this case, reactions occurred within 8 h at 100 C for both activated and deactivated aryl bromides and with electron-poor or electron-neutral aryl chlorides. Reactions of orthosubstituted aryl halides were unusual, providing a mixture of 1- and 3-substituted indoles, but these aryl halides were suitable substrates when the 3-position of the indole was also substituted. The origin of this C- vs. N-arylation is unknown. The Tosoh group has also used this catalyst system for the arylation of the parent pyrrole, indole, and carbazole. They observed that Rb2CO3 was a particularly e ective base [187].
134 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
Tab. 4.5. Reactions of aryl halides with azoles catalyzed by Pd(0)/P(t-Bu)3
Entry Aryl Halide |
Azole |
Product |
Cond.a |
Yieldb (%) |
1 |
|
|
4 % Pd |
72 |
|
|
|
12 h |
|
2 |
3 % Pd |
88 |
|
12 h |
|
4 |
3 % Pd |
83 |
|
6 h |
|
5 |
3 % Pd |
77 |
|
6 h |
|
6 |
5 % Pd |
64 |
|
12 h |
|
a Reactions were run with 1 mmol of azole in 1–2 mL of toluene at |
|
|
100 C. Pd(dba)2 used in combination with 0.8–1.0 equiv. of ligand/Pd. |
|
|
b Isolated yields are an average of at least two runs. |
|
|
Old, Harris, and Buchwald most recently reported the use of their 2-biphenylyl ligands for the arylation of indoles [188]. Similar problems of multiple and non-regiospecific arylation were observed, but some of the ligands alleviated these problems. NaOtBu was the most useful base with these ligands, but K3PO4 could also be used with base-sensitive functionalities. Pd2(dba)3 proved to be superior to Pd(OAc)2 as precursor. The ligands in Chart 5 proved most e ective for these reactions. Ligands 19 and 20 gave the highest rates, and ligand 21 was most e ective in generating the N-aryl indole rather than the products of C3arylation in reactions of 2- and 7-substituted indoles.
Beletskaya has shown that the reaction of benzotriazoles with aryl halides catalyzed by a
Chart 5
4.5 Amination of Base-Sensitive Aryl Halides 135
mixture of dppe (bis(diphenylphosphino)ethane) and copper(I) iodide or copper(II) carboxylates proceeds in good yield in DMF solution with a phase-transfer catalyst [189]. The mechanism of these reactions is unknown, although copper is known to catalyze them. However, it was shown that both copper and palladium are required for these reactions to occur at the N-1 position in high yields. Similar results were observed in aqueous solution with aryliodonium salts as electrophiles [131].
ð25Þ
A remarkable process was recently reported by Mori in which dinitrogen is the source of the nucleophile to form anilines, as shown in Eq. (26) [190]. In this chemistry, dinitrogen is used to generate ‘‘titanium nitrogen fixation complexes’’ by reactions of titanium tetrachloride or tetraisopropoxide, lithium metal, trimethylsilyl chloride, and dinitrogen. These complexes are then allowed to react with the aryl halide and a palladium catalyst to generate a mixture of the aryland diarylamine. In general, palladium ligated by DPPF gave higher yields of the arylamine product, which were as high as 77 % when using 4-biphenyl bromide and 80 % when using 1-naphthyl triflate.
ð26Þ
4.5
Amination of Base-Sensitive Aryl Halides
The use of a strong base in the palladium-catalyzed amination of aryl halides precludes the use of many substrates, such as those with aromatic nitro groups or enolizable hydrogens, esters other than tert-butyl esters, and many substrates with base-sensitive stereochemistry such as some protected amino acids and heterocyclic substrates [191]. Thus, conditions that employ milder bases are required. A solution that involves reaction temperatures as low as those used for reactions conducted with sodium tert-butoxide has not been developed. However, carbonate and phosphate bases can be used with certain catalysts at reaction temperatures comparable to those of reactions involving the firstand second-generation catalysts.
ð27Þ
1364 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
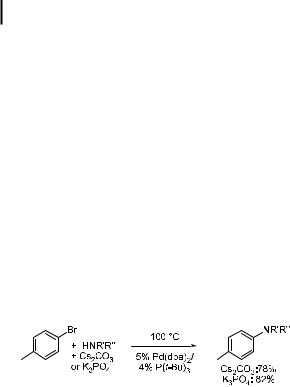
As discussed above, the reactions of amines with aryl triflates instead of aryl halides allow the use of Cs2CO3 as the base. Further, reactions of activated aryl halides and some heteroaryl halides can be conducted with Cs2CO3 as the base instead of tert-butoxide, without requiring a change of catalyst [105, 191]. However, reactions of unactivated aryl halides with this weaker base require a di erent catalyst. A solution to the problem of coupling unactivated aryl halides in the presence of base-sensitive functionalities began with the use of one of Kumada’s phosphino ether ligands (Eq. (27)) [105, 112]. Catalysts containing Kumada’s P,O ligand allowed aminations of unactivated aryl halides in the presence of Cs2CO3 as base. More recently, Buchwald has used the biaryl ligands 13–15 for the amination of aryl halides with Cs2CO3 and the less expensive and less toxic K3PO4 [156]. The catalyst containing commercially available P(tBu)3 also allows the reaction of unactivated aryl halides with secondary amines in the presence of Cs2CO3 or K3PO4 as the base, as shown in Eq. (28)
[163].Thus, simple tert-butyl monophosphines appear to generate active catalysts for reactions involving weaker bases, albeit at temperatures in the range 85–100 C. Presumably the amine can coordinate to the three-coordinate, 14-electron, monophosphine intermediate formed after oxidative addition of aryl halides to complexes with hindered monophosphines [122, 192].
ð28Þ
4.6
Applications of the Amination Chemistry
Studies on the applications of the amination chemistry have begun to emerge. The results demonstrate the utility of the amination in the construction of complex, biologically active molecules, in the synthesis of electronically important structures, and in the synthesis of ligands for other catalytic chemistry.
4.6.1
Synthesis of Biologically Active Molecules
Stoichiometric, palladium-mediated cyclization was used in natural product synthesis by Boger a number of years ago, as was noted in the introduction. More recently, an intramolecular palladium-catalyzed amination of a heteroaromatic halide has been used as a step in the synthesis of an a-carboline natural product analogue [193]. As discussed above, the diphenylhydrazone arylation can also be used for nitrogen heterocycle synthesis [183].
4.6.1.1 Arylation of Secondary Alkylamines
N-Arylpiperazines are common substructures of molecules that influence various biological processes and, therefore, the arylation of monoprotected and the single arylation of unprotected piperazines has been studied. As discussed above, the monoarylation of piperazines

4.6 Applications of the Amination Chemistry 137
Fig. 2. The disconnection used to form hydroxyitraconazole.
was the reaction for which Nishiyama, Koie, et al. initially applied catalysts bearing P(tBu)3 as a ligand to obtain high turnover numbers at high temperatures [136]. Two other reports of piperazine arylation have appeared, one of a Boc-protected piperazine [194] and one of an unprotected piperazine (the original P(o-tolyl)3/Pd(0) catalyst system) [195]. Morita has used the arylation of piperazines as a crucial step in the synthesis of a metabolite of aripiprazole, as shown in Eq. (29) [196]. This reaction involved the use of a tetrasubstituted dichlorobromoarene as substrate to give a product that could be readily converted to the final target. Researchers at Sepracor have conducted the amination of an N-aryl aryl triazolone with a piperazine to generate the two enantiomeric versions of hydroxyitraconazole (Figure 2) [197]. Finally, Schmid reported the use of a piperidine or morpholine group installed by palladium chemistry as a directing and then leaving group in a concise synthesis of raloxifene. Reaction of the cyclic secondary amines with 2-bromobenzo[b]thiophene generated a structure that could be acylated and then reacted at the amino position to deliver an aryl Grignard for synthesis of a 2-aryl benzothiophene [198].
ð29Þ
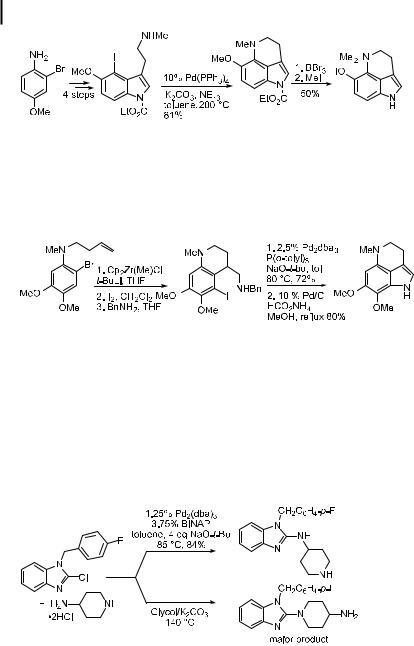
Buchwald has used the intramolecular reaction of acyclic secondary amines with an aryl halide in the total synthesis or the formal total synthesis of a series of tetrahydropyrroloquinolines using palladium-catalyzed amination [199]. A brief description of the methods employed is provided in Eqs. (30) and (31). The approach in Eq. (30) involves formation of the six-membered ring system by means of a palladium-catalyzed intramolecular amination. The reaction conditions contained K2CO3 as the base for the cyclization and involved high temperatures. However, the use of NaOtBu, which might have allowed reaction at lower temperatures, led to cleavage of the carbamate. The cleavage products apparently inhibited catalyst activity.
138 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
ð30Þ
A second approach involved formation of the indole five-membered ring by amination chemistry and the six-membered ring by Zr-benzyne chemistry (Eq. (31)). In this case, the optimal cyclization conditions could be employed, and the reaction temperature was lower. The product of the cyclization is an intermediate in the previous total syntheses of makaluvamine C and of damirones A and B.
ð31Þ
4.6.1.2 Arylation of Primary Alkylamines
Several reports on the application of the intermolecular arylation of primary alkylamines have appeared. For example, the reaction of primary amines with chloro-1,3-azoles has been used to produce the H-1-antihistaminic norastemizole [200]. As shown in Eq. (32), the selectivity of the palladium chemistry is controlled by the steric properties of the amines. This property creates selectivity that complements the thermal chemistry, which is dictated by the amine nucleophilicity. The same researchers have also shown that this high selectivity for arylation of primary over secondary amines with catalysts containing BINAP as ligand allows the rapid assembly of other multiamino-based structures [201].
ð32Þ
Chida has coupled glycosylamines with 6-chloropurines to prepare models of spicamycin and septacidin, two Streptomyces metabolites that show antitumor activity [202]. As shown in Eq. (33), 5 mol % catalyst was used, and the reaction temperature was high (140 C). Nevertheless, good yields of the desired N-aryl glycosylamine were obtained when BINAP was used as the ligand, NaOtBu as the base, and either MPM or SEM as the N9 protective group.
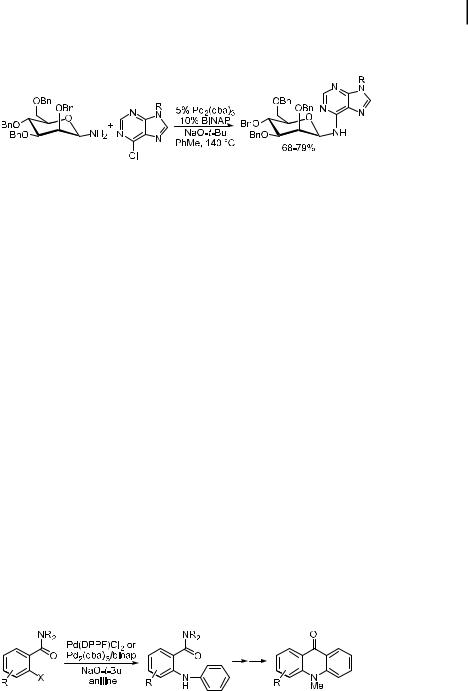
4.6 Applications of the Amination Chemistry 139
The gluco isomer was also amenable to the palladium-catalyzed arylation, although two anomers were obtained as products.
ð33Þ
Recently, the arylation of several specific primary amines have been studied because of the potential biological relevance of the products or products further downstream in a synthetic sequence. For example, cyclopropylamine was shown to be a viable substrate for the coupling under standard conditions [203]. Reactions of 7-azabicyclo[2.2.1]heptane have also been conducted [204] under relatively standard conditions, but with bis(imidazol-2-ylidene) as ligand. Complexes of this ligand and DPPF showed similar catalytic activities, which proved to be superior to those of most bis(phosphine)s. ortho-Halo anilines were also studied, in this case to provide access to carbolines after use of the halogen as a means of e ecting cyclizations by an electrophilic or reductive CaC bond formation with the other N-aryl group [205].
In other cases, unusual aryl halides have been used. Reactions of morpholine and aminopyridine with iodoarylporphyrins have been reported [206]. These reactions take advantage of the ability of chelating ligands to provide activity and were conducted with either DPPF or BINAP as the ligand, depending on the amine substrate. Protected benzimidazoles have been used to prepare (benzimidazolyl)piperazines that show a nity for the 5-HT1A receptor. Yields varied with the amine, but catalysts bearing DPPP or BINAP gave su cient product for biological evaluation [207, 208].
The palladium-catalyzed formation of diarylamines has been used in several contexts to form molecules of biological relevance. The ability to prepare haloarenes selectively by an ortho-metalation–halogenation sequence allows the selective delivery of an amino group to a substituted aromatic structure. Snieckus has used directed metalation to form aryl halides that were subsequently allowed to react with anilines to form diarylamines (Eq. (34)) [209]. Frost and Mendonc¸a have reported an iterative strategy to prepare, by the palladiumcatalyzed chemistry, amides and sulfonamides that may act as peptidomimetics. Diarylamine units were constructed using the DPPF-ligated palladium catalysts, and the products were then acylated or sulfonated with 4-bromobenzoyl or arylsulfonyl chlorides [210]. Lemie`re has coupled primary arylamines with 4-chloro-3(2H)-pyridazinones to form compounds with possible analgesic and antiinflammatory properties.
ð34Þ
Several recent papers have reported the palladium-catalyzed formation of diarylamines to prepare nucleosides of damaged DNA. Sigurdsson, Hopkins et al. reported the formation of
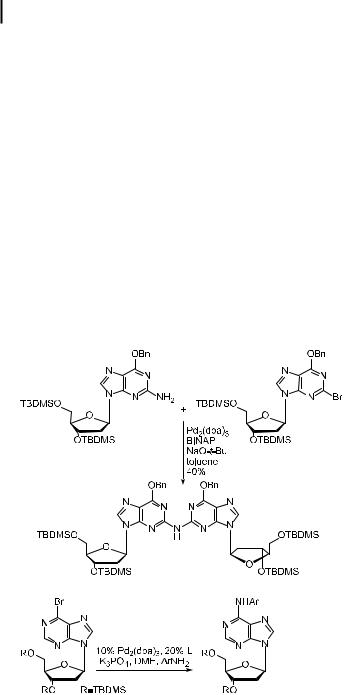
1404 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
a precursor to an interstrand cross-link by the reaction in Eq. (35) [211]. Improved yields were reported by De Riccardis and Johnson when using Cs2CO3. Lakshman reported the reaction of bromonucleosides with amines shown in Eq. (36) as a route to the DNA adducts of carcinogenic aminobiphenyls [212]. In this case, a number of di erent reaction conditions were investigated, and those involving K3PO4 and the P,N ligand 13 proved most e ective for the transformation, albeit with high catalyst loadings. Johnson et al. have also recently reported several preparations of carcinogenic amine adducts of 20-deoxyguanosine and 20-deoxyadenosine. In one case, they allowed protected derivatives of dG or dA to react with o-nitroaryl bromides or triflates, and in another case they coupled 20-deoxy-2-bromoinosine with several di erent amines. The latter method o ered more flexibility because of the greater generality of the procedures involving the reaction of amines with electron-deficient aryl halides [213, 214]. This general strategy was also followed by Wang and Rizzo [215]. An 8-bromo-20-deoxyguanosine derivative was coupled to the food mutagen 2-amino-3- methylimadazo[4,5- f ]quinoline (IQ). To obtain satisfactory yields, they used hexamethyldisilazide as the base and BINAP as the ligand. Several other arylamines and benzylamine were successfully coupled to this guanosine. Finally, Arterburn et al. have recently reported the nickel-catalyzed amination of a nucleobase [216]. They conducted a series of reactions that coupled amino heterocycles and amines tethered to piperazine and piperidine in the presence of a nickel catalyst bearing DPPF as the ligand.
ð35Þ
ð36Þ
An interesting application of the amination in carbohydrate chemistry has been reported by Buchwald and Seeberger [217]. In this work, the amination chemistry was used as a de-
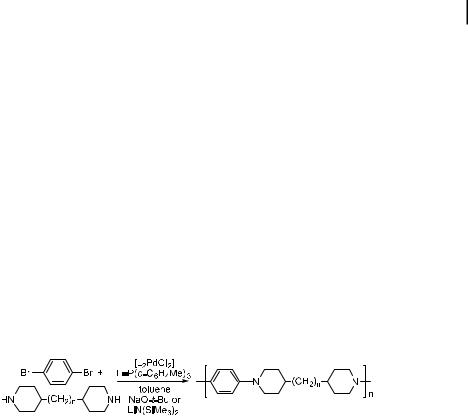
4.6 Applications of the Amination Chemistry 141
protection method for 4-halo benzyl ethers. After the amination, a Lewis or protic acid will readily induce cleavage of the benzyl group. The di erential reactivity of aryl chlorides, bromides, and iodides allows each of the halobenzyl groups to be deprotected selectively. The iodobenzyl protective group reacts with amines more readily than the other halides and, therefore, can be deprotected first. The bromobenzyl group is the next most reactive toward coupling with amine and subsequent deprotection, and the chlorobenzyl group is the least reactive. The halobenzyl substituents, therefore, serve as orthogonal protective groups to PMB, TIPS, acetyl, and simple benzyl substituents.
4.6.2
Applications in Materials Science
Arylamines display electronic properties that are favorable for materials science. In particular, they are readily oxidized to the aminium form, and this oxidation leads to conductivity in polyanilines, hole-transport properties in triarylamines, stable polyradicals with lowenergy or ground-state high-spin states, and the potential to conduct electrochemical sensing. The high yields of the palladium-catalyzed formation of diand triarylamines has allowed ready access to these materials as both small molecules and discrete oligomeric or polymeric macromolecules.
ð37Þ
4.6.2.1 Polymer Synthesis
Several reports on the synthesis of polymeric arylamines by palladium-catalyzed chemistry have appeared. One group used the initial amination of aryl halides with dialkylamines to prepare arylamine polymers [218] by coupling a bifunctional diamine and a dihaloarene, as shown in Eq. (37). The highest molecular weights achieved were in the range 5000–6000, indicating an average of 20 monomers each in a chain. Subsequent to this paper, the same group published work with BINAP and P(tBu)3 as ligands instead of P(o-tolyl)3. Several surprising results were obtained, including higher molecular weights when using P(o-tolyl)3 than when using P(tBu)3, despite the higher yields for the formation of triarylamines in small molecule studies on triarylamine synthesis when using P(tBu)3 [137, 163, 219]. The molecular compositions of these materials were not characterized in detail. Branching and end-capping as a result of phosphine incorporation [220], molecular weight limiting cyclization [219], and precipitation of the polymer may account for these results.
More recently, Buchwald has reported the polymerization of the monomer in Eq. (38) [220]. This monomer was polymerized at 80 C for 24 h in the presence of a catalyst comprised of Pd2(dba)3 and ligand 14. The polymers generated from this monomer bearing a Boc group are soluble in THF and chloroform with the aid of sonication. After isolation, the Boc group could be removed by thermolysis at 185 C or by protonolysis in air. Emeraldine or the emeraldine salt forms of polyaniline result.
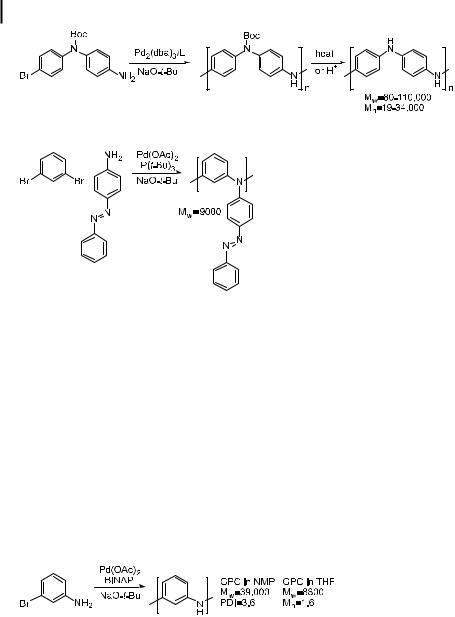
142 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
ð38Þ
ð39Þ
Polymers containing chromophores such as acridine and azobenzene have also been prepared by palladium-catalyzed amination according to two approaches [222–224]. The first approach involved the polymerization of monomers containing the chromophore. For example, 4-aminoazobenzene was condensed with 1,3-dibromobenzene (Eq. (39)) or 4,40- dibromobiphenyl ether to form polymeric materials with Mw values of 9:0 103 and 19 103, respectively. Alternatively, polymers prepared by polymerization of 4-bromostyrene or copolymerization of styrene and 4-bromostyrene were coupled with N-phenyl-4-amino azobenzene. Substitution of the aryl bromides by the amino azobenzene unit was essentially quantitative when using P(tBu)3 as the ligand.
A related approach to the formation of triarylamine-containing polymers involves synthesis of a monomer containing the triarylamine unit and a second functional group that is amenable to polymerization. Grubbs, Marder et al. reported the synthesis of norbornene monomers containing a tethered triarylamine, using DPPF-ligated palladium as a catalyst to form the triarylamine unit. Ruthenium-catalyzed ring-opening metathesis polymerization then forms the triarylamine-containing polymer [225–226].
ð40Þ
Poly(m-aniline) has been prepared by palladium-catalyzed condensation of 1,3-dibromo- benzene and 1,3-phenylene diamine by two di erent research groups (Eq. (40)) [227, 228]. In this case, reactions with BINAP as the ligand gave the highest molecular weights. Kanbara has reported Mw and Mn values in formamide solution of 42,400 and 20,100. Meyer has carefully analyzed the products of these reactions, and these results cast doubt on the molecular weight data obtained by Kanbara, as shown in Eq. (40). First, studies on the reaction yields using small molecules suggest that an Mn value near 7000 should be obtained. Sec-
