
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
|
|
|
References |
163 |
|
|
|
|
|
51 |
D. Baran˜ano, G. Mann, J. F. Hartwig, |
72 |
H. Bryndza, W. Tam, Chem. Rev. 1988, 88, |
|
|
Curr. Org. Chem. 1997, 1, 287–325. |
|
1163–1188. |
|
52 |
D. Cai, J. F. Payack, D. R. Bender, D. L. |
73 |
L. M. Alcazar-Roman, J. F. Hartwig, |
|
|
Hughes, T. R. Verhoeven, P. J. Reider, |
|
2001, unpublished results. |
|
|
J. Org. Chem. 1994, 59, 7180. |
74 |
P. M. Henry, Palladium-Catalyzed |
|
53 |
Y. Uozumi, N. Suzuki, A. Ogiwara, T. |
|
Oxidation of Hydrocarbons, D. Reidel Publ. |
|
|
Hayashi, Tetrahedron 1994, 50, 4293–4302. |
|
Co., Boston, 1980, Vol. 2. |
|
54 |
D. Baran˜ano, J. F. Hartwig, J. Am. |
75 |
B. Cornils, W. A. Herrmann (Eds.), |
|
|
Chem. Soc. 1995, 117, 2937–2938. |
|
Applied Homogeneous Catalysis with |
|
55 |
J. P. Collman, L. S. Hegedus, J. R. |
|
Organometallic Compounds: A Compre- |
|
|
Norton, R. G. Finke, in Principles and |
|
hensive Handbook in Two Volumes, VCH, |
|
|
Applications of Organotransition Metal |
|
New York, 1996. |
|
|
Chemistry; 2nd ed., University Science |
76 |
M. Kawatsura, J. F. Hartwig, J. Am. |
|
|
Books, Mill Valley, 1987, pp 279–354. |
|
Chem. Soc. 2000, 122, 9546–9547. |
|
56 |
R. H. Crabtree, Chem. Rev. 1995, 95, |
77 |
M. R. Gagne´, T. J. Marks, J. Am. Chem. |
|
|
987–1007. |
|
Soc. 1989, 111, 4108–4109. |
|
57 |
S.-Y. Liou, M. Gozin, D. Milstein, J. Am. |
78 |
M. R. Gagne´, S. P. Nolan, T. J. Marks, |
|
|
Chem. Soc. 1995, 117, 9774–9775 and |
|
Organometallics 1990, 9, 1716–1718. |
|
|
references therein. |
79 |
V. M. Arredondo, S. Tian, F. E. |
|
58 |
S. Y. Liou, M. Gozin, D. Milstein, J. |
|
McDonald, T. J. Marks, J. Am. Chem. |
|
|
Chem. Soc., Chem. Commun. 1995, 1965– |
|
Soc. 1999, 121, 3633–3639. |
|
|
1966. |
80 |
A. L. Casalnuovo, J. C. Calabrese, D. |
|
59 |
J. J. Garcia, W. D. Jones, Organometallics |
|
Milstein, J. Am. Chem. Soc. 1988, 110, |
|
|
2000, 19, 5544–5545. |
|
6738–6744. |
|
60 |
B. L. Edelbach, R. J. Lachicotte, W. D. |
81 |
R. Dorta, P. Egli, F. Zurcher, A. Togni, |
|
|
Jones, Organometallics 1999, 18, 4660– |
|
J. Am. Chem. Soc. 1997, 119, 10857–10858. |
|
|
4668. |
82 |
J. P. Wolfe, S. L. Buchwald, J. Am. |
|
61 |
B. L. Edelbach, R. J. Lachicotte, W. D. |
|
Chem. Soc. 1997, 119, 6054–6058. |
|
|
Jones, Organometallics 1999, 18, 4040– |
83 |
E. Brenner, Y. Fort, Tetrahedron Lett. |
|
|
4049. |
|
1998, 39, 5359–5362. |
|
62 |
B. L. Edelbach, D. A. Vicic, R. J. |
84 |
E. Brenner, R. Schneider, Y. Fort, |
|
|
Lachicotte, W. D. Jones, Organometallics |
|
Tetrahedron Lett. 2000, 41, 2881–2884. |
|
|
1998, 17, 4784–4794. |
85 |
C. Desmarets, R. Schneider, Y. Fort, |
|
63 |
M. S. Driver, J. F. Hartwig, J. Am. |
|
Tetrahedron Lett. 2000, 41, 2875–2879. |
|
|
Chem. Soc. 1995, 117, 4708–4709. |
86 |
M. Kosugi, M. Kameyama, T. Migita, |
|
64 |
M. S. Driver, J. F. Hartwig, J. Am. |
|
Chem. Lett. 1983, 927–928. |
|
|
Chem. Soc. 1996, 118, 7217–7218. |
87 |
M. Kosugi, M. Kameyama, H. Sano, T. |
|
65 |
G. Mann, J. F. Hartwig, J. Am. Chem. |
|
Migita, Nippon Kagaku Kaishi 1985, 3, |
|
|
Soc. 1996, 118, 13109–13110. |
|
547–551. |
|
66 |
L. A. Villanueva, K. A. Abboud, J. M. |
88 |
D. L. Boger, J. S. Panek, Tetrahedron Lett. |
|
|
Boncella, Organometallics 1994, 13, 3921– |
|
1984, 25, 3175–3178. |
|
|
3931. |
89 |
D. L. Boger, S. R. Duff, J. S. Panek, M. |
|
67 |
K. Koo, G. L. Hillhouse, Organometallics |
|
Yasuda, J. Org. Chem. 1985, 50, 5782– |
|
|
1995, 14, 4421–4423. |
|
5789. |
|
68 |
P. T. Matsunaga, J. C. Mavropoulos, |
90 |
D. L. Boger, S. R. Duff, J. S. Panek, M. |
|
|
G. L. Hillhouse, Polyhedron 1995, 14, |
|
Yasuda, J. Org. Chem. 1985, 50, 5790– |
|
|
175–185. |
|
5795. |
|
69 |
E. G. Bryan, B. F. G. Johnson, J. Lewis, |
91 |
F. Paul, J. Patt, J. F. Hartwig, J. Am. |
|
|
J. Chem. Soc., Dalton Trans. 1977, 1328– |
|
Chem. Soc. 1994, 116, 5969–5970. |
|
|
1330. |
92 |
A. S. Guram, S. L. Buchwald, J. Am. |
|
70 |
M. S. Driver, J. F. Hartwig, J. Am. |
|
Chem. Soc. 1994, 116, 7901–7902. |
|
|
Chem. Soc. 1996, 118, 4206–4207. |
93 |
M. Kosugi, T. Ogata, M. Terada, H. |
|
71 |
M. D. Fryzuk, C. D. Montgomery, Coord. |
|
Sano, T. Migita, Bull. Chem. Soc. Jpn. |
|
|
Chem. Rev. 1989, 95, 1–40. |
|
1985, 58, 3657–3658. |
|

164 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
94 |
A. Carpita, R. Rossi, B. Scamuzzi, |
116 |
K. Takagi, Y. Sakakibara, Chem. Lett. |
|
Tetrahedron Lett. 1989, 30, 2699–2702. |
|
1989, 1957–1958. |
95 |
S. I. Murahashi, M. Yamamura, K. |
117 |
S. Cacchi, P. G. Ciattini, E. Morera, G. |
|
Yanagisawa, N. Mita, K. Kondo, J. Org. |
|
Ortar, Tetrahedron Lett. 1986, 27, 3931– |
|
Chem. 1979, 44, 2408–2417. |
|
3934. |
96 |
T. Migita, T. Shimiza, Y. Asami, J. |
118 |
J. Louie, M. S. Driver, B. C. Hamann, |
|
Shiobara, Y. Kato, M. Kosugi, Bull. |
|
J. F. Hartwig, J. Org. Chem. 1997, 62, |
|
Chem. Soc. Jpn. 1980, 53, 1385. |
|
1268–1273. |
97 |
S. E. Tunney, J. K. Stille, J. Org. Chem. |
119 |
J. P. Wolfe, S. L. Buchwald, J. Org. |
|
1987, 52, 748–753. |
|
Chem. 1997, 62, 1264–1267. |
98 |
J. Louie, J. F. Hartwig, Tetrahedron Lett. |
120 |
˚ |
J. Ahman, S. L. Buchwald, Tetrahedron |
|||
|
1995, 36, 3609–3612. |
|
Lett. 1997, 38, 6363–6366. |
99 |
A. S. Guram, R. A. Rennels, S. L. |
121 |
Y. Torisawa, T. Nishi, J. Minamikawa, |
|
Buchwald, Angew. Chem. Int. Ed. Engl. |
|
Bioorg. Med. Chem. Lett. 2000, 10, 2489– |
|
1995, 34, 1348–1350. |
|
2491. |
100 |
J. P. Wolfe, R. A. Rennels, S. L. Buchwald, |
122 |
F. Paul, J. Patt, J. F. Hartwig, Organo- |
|
Tetrahedron 1996, 52, 7525–7546. |
|
metallics 1995, 14, 3030–3039. |
101 |
M. S. Driver, J. F. Hartwig, J. Am. |
123 |
S. Wagaw, S. L. Buchwald, J. Org. Chem. |
|
Chem. Soc. 1997, 119, 8232–8245. |
|
1996, 61, 7240–7241. |
102 |
J. F. Hartwig, J. Am. Chem. Soc. 1996, |
124 |
J. B. Arterburn, K. V. Rao, R. Ramdas, |
|
118, 7010–7011. |
|
B. R. Dible, Org. Lett. 2001, 3, 1351–1354. |
103 |
T. Hayashi, M. Konoshi, Y. Kobori, M. |
125 |
D. R. Coulson, Inorg. Synth. 1990, 28, |
|
Kumada, T. Higuchi, K. Hirotsu, J. Am. |
|
107–109. |
|
Chem. Soc. 1984, 106, 158–163. |
126 |
M. Watanabe, T. Yamamoto, M. |
104 |
J. P. Wolfe, S. Wagaw, S. L. Buchwald, |
|
Nishiyama, Chem. Commun. 2000, 133– |
|
J. Am. Chem. Soc. 1996, 118, 7215–7216. |
|
134. |
105 |
J. P. Wolfe, S. L. Buchwald, Tetrahedron |
127 |
Y. D. Ward, V. Farina, Tetrahedron Lett. |
|
Lett. 1997, 38, 6359–6362. |
|
1996, 37, 6993–6996. |
106 |
B. C. Hamann, J. F. Hartwig, J. Am. |
128 |
C. A. Willoughby, K. T. Chapman, |
|
Chem. Soc. 1997, 119, 12382–12383. |
|
Tetrahedron Lett. 1996, 37, 7181–7184. |
107 |
B. C. Hamann, J. F. Hartwig, J. Am. |
129 |
P. H. H. Hermkens, H. C. J. |
|
Chem. Soc. 1998, 120, 3694–3703. |
|
Ottenheijm, D. Rees, Tetrahedron 1997, |
108 |
J. P. Wolfe, S. L. Buchwald, J. Org. |
|
52, 4527–4554. |
|
Chem. 2000, 65, 1144–1157. |
130 |
B. Witulski, S. Senft, A. Thum, Synlett |
109 |
S. Wagaw, R. A. Rennels, S. L. |
|
1998, 504–505. |
|
Buchwald, J. Am. Chem. Soc. 1997, 119, |
131 |
I. P. Beletskaya, D. V. Davydov, M. |
|
8451–8458. |
|
Morenomanas, Tetrahedron Lett. 1998, 39, |
110 |
K. Rossen, P. J. Pye, A. Maliakal, R. P. |
|
5621–5622. |
|
Volante, J. Org. Chem. 1997, 62, 6462– |
132 |
I. Beletskaya, A. Bessmertnykh, R. |
|
6463. |
|
Guilard, Synlett 1999, 9, 1459–1461. |
111 |
J. P. Sadighi, M. C. Harris, S. L. |
133 |
M. Palucki, J. P. Wolfe, S. L. Buchwald, |
|
Buchwald, Tetrahedron Lett. 1998, 39, |
|
J. Am. Chem. Soc. 1997, 119, 3395–3396. |
|
5327–5330. |
134 |
S. Hayden, J. R. J. Sowa, Proc. Catal. Org. |
112 |
J.-F. Marcoux, S. Wagaw, S. L. |
|
React. 1998, 627–632. |
|
Buchwald, J. Org. Chem. 1997, 62, 1568– |
135 |
J. F. Hartwig, S. Richards, D. |
|
1569. |
|
Baran˜ano, F. Paul, J. Am. Chem. Soc. |
113 |
K. Kamikawa, S. Sugimoto, M. Uemura, |
|
1996, 118, 3626–3633. |
|
J. Org. Chem. 1998, 63, 8407–8410. |
136 |
M. Nishiyama, T. Yamamoto, Y. Koie, |
114 |
G. Wullner, H. Jansch, S. Kannenberg, |
|
Tetrahedron Lett. 1998, 39, 617–620. |
|
F. Schubert, G. Boche, Chem. Commun. |
137 |
T. Yamamoto, M. Nishiyama, Y. Koie, |
|
1998, 1509–1510. |
|
Tetrahedron Lett. 1998, 39, 2367–2370. |
115 |
M. Prashad, B. Hu, Y. Lu, R. Draper, D. |
138 |
I. R. Butler, W. R. Cullen, T. J. Kim, |
|
Har, O. Repic, T. J. Blacklock, J. Org. |
|
S. J. Rettig, J. Trotter, Organometallics |
|
Chem. 2000, 65, 2612–2614. |
|
1985, 4, 972–980. |
|
|
|
References |
165 |
|
|
|
|
|
139 |
W. R. Cullen, T. J. Kim, F. W. B. |
|
Leeuwen, K. Goubitz, J. Fraanje, |
|
|
Einstein, T. Jones, Organometallics 1983, |
|
Organometallics 1995, 14, 3081–3089. |
|
|
2, 714–719. |
160 |
M. H. Ali, S. L. Buchwald, J. Org. Chem. |
|
140 |
H. Blaser, F. Spindler, Chimia 1997, 51, |
|
2001, 66, 2560–2565. |
|
|
297–299. |
161 |
X. Bei, T. Uno, J. Norris, H. W. Turner, |
|
141 |
A. Togni, C. Breutel, M. C. Soares, N. |
|
W. H. Weinberg, A. S. Guram, |
|
|
Zanetti, T. Gerfin, V. Gramlich, F. |
|
Organometallics 1999, 18, 1840–1853. |
|
|
Spindler, G. Rihs, Inorg. Chim. Acta |
162 |
X. Bei, A. S. Guram, H. W. Turner, |
|
|
1994, 222, 213–224. |
|
W. H. Weinberg, Tetrahedron Lett. 1999, |
|
142 |
A. Togni, C. Breutel, A. Schnyder, F. |
|
40, 1237–1240. |
|
|
Spindler, H. Landert, A. Tijani, J. Am. |
163 |
J. F. Hartwig, M. Kawatsura, S. I. |
|
|
Chem. Soc. 1994, 116, 4062–4066. |
|
Hauck, K. H. Shaughnessy, L. M. |
|
143 |
M. Beller, T. H. Reirmeier, C. |
|
Alcazar-Roman, J. Org. Chem. 1999, 64, |
|
|
Reisinger, W. A. Herrman, Tetrahedron |
|
5575–5580. |
|
|
Lett. 1997, 38, 2073–2074. |
164 |
S. I. Hauck, J. F. Hartwig, unpublished |
|
144 |
S. Saito, M. Sakai, N. Miyaura, Tetra- |
|
results. |
|
|
hedron Lett. 1996, 37, 2993–2996. |
165 |
J. Huang, G. Grasa, S. P. Nolan, Org. |
|
145 |
A. F. Indolese, Tetrahedron Lett. 1997, 38, |
|
Lett. 1999, 1, 1307–1309. |
|
|
3513–3516. |
166 |
G. A. Grasa, M. S. Viciu, J. K. Huang, |
|
146 |
S. Saito, S. Oh-tani, N. Miyaura, J. Org. |
|
S. P. Nolan, J. Org. Chem. 2001, 66, |
|
|
Chem 1997, 62, 8024–8030. |
|
7729–7737. |
|
147 |
J. Miller, R. Farrell, Tetrahedron Lett. |
167 |
S. Stauffer, S. I. Hauck, S. Lee, J. |
|
|
1998, 6441–6444. |
|
Stambuli, J. F. Hartwig, Org. Lett. 2000, |
|
148 |
J.-C. Galland, M. Savignac, J.-P. Geneˆt, |
|
2, 1423–1426. |
|
|
Tetrahedron Lett. 1999, 40, 2323–2326. |
168 |
J. K. Huang, H. J. Schanz, E. D. |
|
149 |
B. H. Lipshutz, P. A. Blomgren, J. Am. |
|
Stevens, S. P. Nolan, Organometallics |
|
|
Chem. Soc. 1999, 121, 5819–5820. |
|
1999, 18, 2370–2375. |
|
150 |
B. C. Hamann, J. F. Hartwig, J. Am. |
169 |
S. Caddick, F. G. N. Cloke, G. K. B. |
|
|
Chem. Soc. 1998, 120, 7369–7370. |
|
Clentsmith, P. B. Hitchcock, D. |
|
151 |
D. Badone, R. Cecchi, U. Guzzi, J. Org. |
|
McKerrecher, L. R. Titcomb, M. R. V. |
|
|
Chem. 1992, 57, 6321–6323. |
|
Williams, J. Organomet. Chem. 2001, 617– |
|
152 |
Y. Kubota, S. Nakada, Y. Sugi, Synlett |
|
618, 635–639. |
|
|
1998, 183–185. |
170 |
G. Y. Li, Angew. Chem. Int. Ed. 2001, 40, |
|
153 |
T. Hayashi, T. Mise, M. Fukishima, M. |
|
1513–1516. |
|
|
Kagotani, N. Nagashima, Y. Hamada, A. |
171 |
G. Y. Li, G. Zheng, A. F. Noonan, J. Org. |
|
|
Matsumoto, S. Kawakami, M. Konoshi, |
|
Chem. 2001, 66, 8677–8681. |
|
|
K. Yamamoto, M. Kumada, Bull. Chem. |
172 |
L. Djakovitch, M. Wagner, K. Kohler, J. |
|
|
Soc. Jpn. 1980, 53, 1138–1151. |
|
Organomet. Chem. 1999, 592, 225–234. |
|
154 |
T. Hayashi, M. Konoshi, M. Fukushima, |
173 |
B. H. Lipshutz, H. Ueda, Angew. Chem. |
|
|
T. Mise, M. Kagotani, M. Tajika, M. |
|
Int. Ed. 2000, 39, 4492–4494. |
|
|
Kumada, J. Am. Chem. Soc. 1982, 104, |
174 |
B. H. Yang, S. L. Buchwald, Org. Lett. |
|
|
180–186. |
|
1999, 1, 35–37. |
|
155 |
S. Vyskocil, M. Smrcina, P. Kocovsky, |
175 |
W. Shakespeare, Tetrahedron Lett. 1999, |
|
|
Tetrahedron Lett. 1998, 39, 9289–9292. |
|
40, 2035–2038. |
|
156 |
D. W. Old, J. P. Wolfe, S. L. Buchwald, |
176 |
J. Yin, S. L. Buchwald, Org. Lett. 2000, 2, |
|
|
J. Am. Chem. Soc. 1998, 120, 9722–9723. |
|
1101–1104. |
|
157 |
J. P. Wolfe, S. Wagaw, J.-F. Marcoux, |
177 |
S. D. Edmondson, A. Mastracchio, E. R. |
|
|
S. L. Buchwald, Acc. Chem. Res. 1998, 31, |
|
Parmee, Org. Lett. 2000, 2, 1109–1112. |
|
|
805–818. |
178 |
S. Jaime-Figueroa, Y. Liu, J. M. |
|
158 |
J. P. Wolfe, H. Tomori, J. P. Sadighi, |
|
Muchowski, D. G. Putman, Tetrahedron |
|
|
J. J. Yin, S. L. Buchwald, J. Org. Chem. |
|
Lett. 1998, 39, 1313–1316. |
|
|
2000, 65, 1158–1174. |
179 |
G. Mann, D. Baran˜ano, J. F. Hartwig, |
|
159 |
M. Kranenburg, Y. E. M. van der |
|
A. L. Rheingold, I. A. Guzei, J. Am. |
|
|
Burgt, P. C. J. Kamer, P. W. N. M. van |
|
Chem. Soc. 1998, 120, 9205–9219. |
|

166 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
180 |
˚ |
201 |
Y. P. Hong, C. H. Senanayake, T. J. |
J. P. Wolfe, J. Ahman, J. P. Sadighi, |
|||
|
R. A. Singer, S. L. Buchwald, Tetra- |
|
Xiang, C. P. Vandenbossche, G. J. |
|
hedron Lett. 1997, 38, 6367–6370. |
|
Tanoury, R. P. Bakale, S. A. Wald, |
181 |
C. Bolm, Angew. Chem. Int. Ed. Engl. 1993, |
|
Tetrahedron Lett. 1998, 39, 3121–3124. |
|
32, 232–233. |
202 |
N. Chida, T. Suzuki, S. Tanaka, I. |
182 |
C. Bolm, J. P. Hildebrand, J. Org. Chem. |
|
Yamada, Tetrahedron Lett. 1999, 40, 2573– |
|
2000, 65, 169–175. |
|
2576. |
183 |
S. Wagaw, B. H. Yang, S. L. Buchwald, |
203 |
W. Cui, R. N. Loeppky, Tetrahedron 2001, |
|
J. Am. Chem. Soc. 1998, 120, 6621–6622. |
|
57, 2953–2956. |
184 |
J. F. Hartwig, Angew. Chem. Int. Ed. 1998, |
204 |
J. Cheng, M. L. Trudell, Org. Lett. 2001, |
|
37, 2090–2093. |
|
3, 1371–1374. |
185 |
S. Wagaw, B. Yang, S. L. Buchwald, J. |
205 |
T. Iwaki, Y. Akito, T. Sakamoto, J. Chem. |
|
Am. Chem. Soc. 1999, 121, 10251–10263. |
|
Soc., Perkin Trans. 1 1999, 1505–1510. |
186 |
Z. Wang, R. T. Skerlj, G. J. Bridger, |
206 |
M. M. Khan, H. Ali, J. E. van Lier, |
|
Tetrahedron Lett. 1999, 40, 3543–3546. |
|
Tetrahedron Lett. 2001, 42, 1615–1617. |
187 |
M. Watanabe, M. Nishiyama, T. |
207 |
M. L. Lopez-Rodriguez, A. Viso, B. |
|
Yamamoto, Y. Koie, Tetrahedron Lett. |
|
Benhamu, J. L. Rominguera, M. Murcia, |
|
2000, 41, 481–483. |
|
Bioorg. Med. Chem. Lett. 1999, 9, 2339– |
188 |
D. W. Old, M. C. Harris, S. L. |
|
2342. |
|
Buchwald, Org. Lett. 2000, 2, 1403–1406. |
208 |
M. L. Lopez-Rodriguez, B. Benhamu, |
189 |
I. P. Beletskaya, D. V. Davydov, M. |
|
D. Ayala, J. L. Rominguera, M. Murcia, |
|
Morenomanas, Tetrahedron Lett. 1998, 39, |
|
J. A. Ramos, A. Viso, Tetrahedron 2000, |
|
5617–5620. |
|
56, 3245–3253. |
190 |
K. Hori, M. Mori, J. Am. Chem. Soc. |
209 |
S. L. Macneil, M. Gray, L. E. Briggs, J. J. |
|
1998, 120, 7651–7652. |
|
Li, V. Snieckus, Synlett 1998, 419–421. |
191 |
J. Kosmrlj, B. U. W. Maes, G. L. F. |
210 |
C. G. Frost, P. Mendonc¸a, Chem. Lett. |
|
Lemiere, A. Haemers, Synlett 2000, 1581– |
|
1997, 1159–1160. |
|
1584. |
211 |
E. A. Harwood, S. T. Sigurdsson, |
192 |
R. A. Widenhoefer, S. L. Buchwald, |
|
N. B. F. Edfeldt, B. R. Reid, P. B. |
|
Organometallics 1996, 15, 2755–2763. |
|
Hopkins, J. Am. Chem. Soc. 1999, 121, |
193 |
A. Abouabdellah, R. Dodd, Tetrahedron |
|
5081–5082. |
|
Lett. 1998, 39, 2119–2122. |
212 |
M. K. Lakshman, J. C. Keeler, J. H. |
194 |
F. Kerrigan, C. Martin, G. H. Thomas, |
|
Hilmer, J. Q. Martin, J. Am. Chem. Soc. |
|
Tetrahedron Lett. 1998, 39, 2219–2222. |
|
1999, 121, 6090–6091. |
195 |
S. Zhao, A. K. Miller, J. Berger, L. A. |
213 |
R. R. Bonala, I. G. Shishkina, F. Johnson, |
|
Flippin, Tetrahedron Lett. 1996, 37, 4463– |
|
Tetrahedron Lett. 2000, 41, 7281–7284. |
|
4466. |
214 |
F. De Riccardis, R. Bonala, F. Johnson, |
196 |
S. Morita, K. Kitano, J. Matsubara, T. |
|
J. Am. Chem. Soc. 1999, 121, 10453–10460. |
|
Ohtani, Y. Kawano, K. Otsubo, M. |
215 |
Z. W. Wang, C. J. Rizzo, Org. Lett. 2001, |
|
Uchida, Tetrahedron 1998, 54, 4811– |
|
3, 565–568. |
|
4818. |
216 |
J. B. Arterburn, M. Pannala, A. M. |
197 |
G. J. Tanoury, C. H. Senanayake, R. |
|
Gonzalez, Tetrahedron Lett. 2001, 42, |
|
Hett, A. M. Kuhn, D. W. Kessler, S. A. |
|
1475–1477. |
|
Wald, Tetrahedron Lett. 1998, 39, 6845– |
217 |
O. J. Plante, S. L. Buchwald, P. H. |
|
6848. |
|
Seeberger, J. Am. Chem. Soc. 2000, 122, |
198 |
D. A. Bradley, A. G. Godfrey, C. R. |
|
7148–7149. |
|
Schmid, Tetrahedron Lett. 1999, 40, 5155– |
218 |
T. Kanbara, A. Honma, K. Hasegawa, |
|
5159. |
|
Chem. Lett. 1996, 1135–1136. |
199 |
A. J. Peat, S. L. Buchwald, J. Am. Chem. |
219 |
F. E. Goodson, S. I. Hauck, J. F. |
|
Soc. 1996, 118, 1028–1030. |
|
Hartwig, J. Am. Chem. Soc. 1999, 121, |
200 |
Y. P. Hong, G. J. Tanoury, H. S. |
|
7527–7539. |
|
Wilkinson, R. P. Bakale, S. A. Wald, |
220 |
X. X. Zhang, J. P. Sadighi, T. W. |
|
C. H. Senanayake, Tetrahedron Lett. 1997, |
|
Mackewitz, S. L. Buchwald, J. Am. |
|
38, 5663–5666. |
|
Chem. Soc. 2000, 122, 7606–7607. |
|
|
|
References |
167 |
|
T. I. Wallow, B. M. Novak, Polym. Prepr. |
|
|
|
221 |
|
Darcos, J. P. Desvergne, D. M. Bassani, |
||
|
1993, 34, 1009. |
|
H. Bouas-Laurent, Tetrahedron Lett. 1998, |
|
222 |
T. Kanbara, T. Imayasu, K. Hasegawa, |
|
39, 4807–4808. |
|
|
Chem. Lett. 1998, 709–710. |
244 |
B. Witulski, Synlett 1999, 8, 1223–1226. |
|
223 |
T. Kanbara, M. Oshima, K. Hasegawa, J. |
245 |
B. Witulski, M. Weber, U. |
|
|
Polym. Sci. Polym. Chem. 1998, 36, 2155– |
|
Bergstrasser, J. P. Desvergne, D. M. |
|
|
2160. |
|
Bassani, H. Bouas-Laurent, Org. Lett. |
|
224 |
T. Kanbara, M. Oshima, T. Imayasu, K. |
|
2001, 3, 1467–1470. |
|
|
Hasegawa, Macromolecules 1998, 31, 8725– |
246 |
I. P. Beletskaya, A. G. Bessermertnykh, |
|
|
8730. |
|
R. Guilard, Tetrahedron Lett. 1997, 38, |
|
225 |
R. H. Grubbs, W. Tumas, Science 1989, |
|
2287–2290. |
|
|
243, 907. |
247 |
N. Montserrat, A. W. Parkins, A. R. |
|
226 |
P. Schwab, R. H. Grubbs, J. W. Ziller, J. |
|
Tomkins, J. Chem. Res., Synop. 1995, 336– |
|
|
Am. Chem. Soc. 1996, 118, 100. |
|
337. |
|
227 |
N. Spetseris, R. E. Ward, T. Y. Meyer, |
248 |
A. Mendiratta, S. Barlow, M. W. Day, |
|
|
Macromolecules 1998, 31, 3158–3161. |
|
S. R. Marder, Organometallics 1999, 18, |
|
228 |
T. Kanbara, K. Izumi, Y. Nakadani, T. |
|
454–456. |
|
|
Narise, K. Hasegawa, Chem. Lett. 1997, |
249 |
K. R. J. Thomas, J. T. Lin, Y. T. Tao, |
|
|
1185–1186. |
|
C. W. Ko, Adv. Mater. 2000, 12, 1949. |
|
229 |
T. Kanbara, Y. Nakadani, K. Hasegawa, |
250 |
S. Thayumanavan, S. Barlow, S. R. |
|
|
Polym. J. 1999, 31, 206–209. |
|
Marder, Chem. Mater. 1997, 9, 3231–3235. |
|
230 |
T. Kanbara, Y. Miyazaki, K. Hasegawa, |
251 |
M. C. Harris, O. Geis, S. L. Buchwald, J. |
|
|
T. Yamamoto, J. Polym. Sci. Polym. Chem. |
|
Org. Chem. 1999, 64, 6019–6022. |
|
|
2000, 38, 4194–4199. |
252 |
M. C. Harris, S. L. Buchwald, J. Org. |
|
231 |
T. Braig, D. C. Muller, M. Gross, K. |
|
Chem. 2000, 65, 5327–5333. |
|
|
Meerholz, O. Nuyken, Macromol. Rapid |
253 |
M. S. Bayerl, T. Braig, O. Nuyken, D. C. |
|
|
Commun. 2000, 21, 583–589. |
|
Muller, M. Gross, K. Meerholz, |
|
232 |
F. E. Goodson, J. F. Hartwig, |
|
Macromol. Rapid Commun. 1999, 20, 224– |
|
|
Macromolecules 1998, 31, 1700–1703. |
|
228. |
|
233 |
F. E. Goodson, T. I. Wallow, B. M. |
254 |
J. Ipaktschi, A. Sharifi, Monatsh. Chem. |
|
|
Novak, Macromolecules 1998, 31, 2047– |
|
1998, 129, 915–920. |
|
|
2056. |
255 |
S. Becker, A. Bohm, K. Mullen, Chem. |
|
234 |
F. E. Goodson, T. I. Wallow, B. M. |
|
Eur. J. 2000, 6, 3984–3990. |
|
|
Novak, J. Am. Chem. Soc. 1997, 119, |
256 |
G. E. Greco, A. I. Popa, R. R. Schrock, |
|
|
12441–12453. |
|
Organometallics 1998, 17, 5591–5593. |
|
235 |
R. A. Singer, J. P. Sadighi, S. L. |
257 |
R. R. Schrock, A. L. Casado, J. T. |
|
|
Buchwald, J. Am. Chem. Soc. 1998, 120, |
|
Goodman, L. C. Liang, P. J. |
|
|
213–214. |
|
Bonitatebus, W. M. Davis, |
|
236 |
J. P. Sadighi, R. A. Singer, S. L. |
|
Organometallics 2000, 19, 5325–5341. |
|
|
Buchwald, J. Am. Chem. Soc. 1998, 120, |
258 |
L. C. Liang, R. R. Schrock, W. M. Davis, |
|
|
4960–4976. |
|
Organometallics 2000, 19, 2526–2531. |
|
237 |
J. Louie, J. F. Hartwig, Macromolecules |
259 |
F. A. Hicks, M. Brookhart, Org. Lett. |
|
|
1998, 31, 6737–6739. |
|
2000, 2, 219–221. |
|
238 |
M. P. Struijk, R. A. Janssen, Synth. |
260 |
I. Cabanal-Duvillard, P. Mangeney, |
|
|
Metals 1999, 103, 2287–2290. |
|
Tetrahedron Lett. 1999, 40, 3877–3880. |
|
239 |
A. Ito, Y. Ono, K. Tanaka, New J. Chem. |
261 |
S. E. Denmark, X. Su, Y. Nishigaichi, |
|
|
1998, 22, 779–781. |
|
D. M. Coe, K.-T. Wong, S. B. D. Winter, |
|
240 |
A. Ito, Y. Ono, K. Tanaka, J. Org. Chem. |
|
J. Y. Choi, J. Org. Chem. 1999, 64, 1958– |
|
|
1999, 64, 8236–8241. |
|
1967. |
|
241 |
A. Ito, Y. Ono, K. Tanaka, Angew. Chem. |
262 |
C. G. Frost, P. Mendonc¸a, Tetrahedron: |
|
|
Int. Ed. 2000, 39, 1072–1075. |
|
Asymmetry 1999, 10, 1831–1834. |
|
242 |
S. I. Hauck, K. V. Lakshmi, J. F. |
263 |
S. Vyskocil, M. Smrcina, P. Kocovsky, |
|
|
Hartwig, Org. Lett. 1999, 1, 2057–2060. |
|
Collect. Czech. Chem. Commun. 1998, 63, |
|
243 |
B. Witulski, Y. Zimmermann, V. |
|
515–519. |
|

168 4 Palladium-Catalyzed Amination of Aryl Halides and Sulfonates
264 |
S. Vyskocil, S. Jaracz, M. Smrcina, |
279 |
J. Louie, F. Paul, J. F. Hartwig, |
|
M. Sticha, V. Hanus, M. Polasek, P. |
|
Organometallics 1996, 15, 2794–2805. |
|
Kocovsky, J. Org. Chem. 1998, 63, 7727– |
280 |
M. S. Driver, J. F. Hartwig, |
|
7737. |
|
Organometallics 1997, 16, 5706–5715. |
265 |
R. A. Singer, S. L. Buchwald, Tetra- |
281 |
The use of BINAP in Buchwald’s |
|
hedron Lett. 1999, 40, 1095–1098. |
|
laboratory was initiated by studies on the |
266 |
F. M. Rivas, U. Riaz, S. T. Diver, |
|
kinetic resolution of chiral amines. |
|
Tetrahedron: Asymmetry 2000, 11, 1703– |
282 |
G. Mann, C. Incarvito, A. L. |
|
1707. |
|
Rheingold, J. F. Hartwig, J. Am. Chem. |
267 |
C. Amatore, G. Broeker, A. Jutand, F. |
|
Soc. 1999, 121, 3224–3225. |
|
Khalil, J. Am. Chem. Soc. 1997, 119, |
283 |
M. D. Fryzuk, W. E. Piers, Organo- |
|
5176–5185. |
|
metallics 1990, 9, 986–98. |
268 |
L. M. Alcazar-Roman, J. F. Hartwig, |
284 |
J. B. Cannon, J. Schwartz, J. Am. Chem. |
|
A. L. Rheingold, L. M. Liable-Sands, |
|
Soc. 1974, 96, 2276–2278. |
|
I. A. Guzei, J. Am. Chem. Soc. 2000, 122, |
285 |
J. Evans, J. Schwartz, P. W. Urquhart, |
|
4618–4630. |
|
J. Organomet. Chem. 1974, 81, C37–C39. |
269 |
P. Kocovsky, S. Vyskocil, I. Cisarova, J. |
286 |
R. J. Cross, in The Chemistry of the Metal– |
|
Sejbal, I. Ticlerova´, M. Smrcina, G. C. |
|
Carbon Bond (Eds.: F. R. Hartley, S. |
|
Lloyd-Jones, S. C. Stephen, C. P. Butts, |
|
Patai), John Wiley, New York, 1985, Vol. |
|
M. Murray, V. Langer, J. Am. Chem. Soc. |
|
2, pp 559. |
|
1999, 121, 7714–7715. |
287 |
G. M. Whitesides, J. F. Gaasch, E. R. |
270 |
N. M. Brunkan, P. S. White, M. R. |
|
Stedronsky, J. Am. Chem. Soc. 1972, 94, |
|
Gagne, J. Am. Chem. Soc. 1998, 120, |
|
5258–5270. |
|
11002–11003. |
288 |
J. Zhao, H. Hesslink, J. F. Hartwig, J. |
271 |
J. F. Hartwig, F. Paul, J. Am. Chem. Soc. |
|
Am. Chem. Soc. 2001, 123, 7220–7227. |
|
1995, 117, 5373–5374. |
289 |
W. D. Jones, V. L. Kuykendall, Inorg. |
272 |
E. Negishi, T. Takahashi, K. Akiyoshi, |
|
Chem 1991, 30, 2615–2622. |
|
J. Chem Soc., Chem. Commun. 1986, 1338. |
290 |
J. P. Wolfe, S. L. Buchwald, Angew. |
273 |
C. Amatore, F. Pfluger, Organometallics |
|
Chem. Int. Ed. 1999, 38, 2413–2416. |
|
1990, 9, 2276–2282. |
291 |
J. M. Brown, P. J. Guiry, Inorg. Chim. |
274 |
C. Amatore, A. Jutand, A. Suarez, J. Am. |
|
Acta 1994, 220, 249–259. |
|
Chem. Soc. 1993, 115, 9531–9541. |
292 |
Y. Guari, G. P. F. van Strijdonck, M. D. |
275 |
J. K. Stille, K. S. Y. Lau, Acc. Chem. Res. |
|
Boele, J. N. H. Reek, P. C. J. Kamer, |
|
1977, 10, 434–442. |
|
P. W. N. M. van Leeuwen, Chem. Eur. J. |
276 |
A. Roy, J. F. Hartwig, J. Am. Chem. Soc. |
|
2001, 7, 475–482. |
|
2001, 123, 1232–1233. |
293 |
L. M. Alcazar-Roman, J. F. Hartwig, J. |
277 |
R. A. Widenhoefer, S. L. Buchwald, |
|
Am. Chem. Soc. 2001, 123, 12905–12906. |
|
Organometallics 1996, 15, 3534–3542. |
294 |
C. Amatore, A. Jutand, A. Suarez, J. |
278 |
R. A. Widenhoefer, H. A. Zhong, S. L. |
|
Am. Chem. Soc. 1993, 115, 9531–9541. |
|
Buchwald, Organometallics 1996, 15, |
295 |
C. Amatore, A. Jutand, J. Organomet. |
|
2745–2754. |
|
Chem. 1999, 576, 254–278. |

169
5
From Acetylenes to Aromatics: Novel Routes – Novel Products
Henning Hopf
Abstract
Alkynes have been used as precursors for aromatic compounds since Berthelot converted acetylene to benzene in 1867. The review presents new evidence on the mechanism of this first total synthesis of an aromatic compound. Presumably, its last step involves the thermocyclization of hexa-1,3-dien-5-yne to benzene. Trapping experiments as well as the study of derivatives of this highly unsaturated hydrocarbon show that the reaction takes place via isobenzene intermediates (1,2,4-cyclohexatrienes) at lower temperatures. Under high temperature pyrolysis conditions, carbenes as well as radical routes compete with the pericyclic process. In another recent application of acetylenes in the synthesis of aromatic compounds, the use of the isomeric diethynylbenzenes is discussed, which, depending on the actual substitution pattern, may be used for the construction of long extended p-systems or cyclic derivatives. When the number of triple bond substituents at the aromatic core is increased, subsystems of novel carbon allotropes become available (‘‘graphyne’’). Cyclooligomerization of acetylenes in the presence of cobalt catalysts leads to phenylenes, a class of condensed aromatic compounds hitherto available only with di culty. Debromination of bis(propargyl bromide)-substituted aromatics provides highly reactive intermediates, which cyclodimerize to cyclophanes possessing unsaturated molecular bridges (cyclophynes). Cyclophynes are not only of interest for structural and stereochemical reasons but also as precursors of novel fullerene derivatives.
5.1
Introduction
Based on earlier studies on the total synthesis of benzene (2), which he called the ‘‘keystone of the total aromatic edifice’’, Berthelot in 1867 carried out a remarkable experiment: heating acetylene (1) – which he had prepared from the elements – in a ‘‘bent bell-jar at a temperature where the glass began to soften’’, he noticed the formation of ‘‘polymeric substances’’. When these were subjected to fractional distillation, benzene, styrene, and other aromatic hydrocarbons could be isolated, with 2 constituting approximately half of the product mixture (Scheme 1) [1].
In the long period following Berthelot’s courageous e orts, not only was the thermal tri-
Modern Arene Chemistry. Edited by Didier Astruc
Copyright 8 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30489-4
1705 From Acetylenes to Aromatics: Novel Routes -- Novel Products
Scheme 1. Berthelot’s benzene synthesis.
merization of 1 and many of its derivatives repeated many times (under safer conditions), but it was also shown that this ring-generating oligomerization could be brought about by irradiation with ultraviolet light as well as catalytically in the presence of metals, metal salts, and – in particular – metal p-complexes, notably those of nickel, iron, cobalt, rhodium, and many others [2]. Many of these metal-mediated transformations, which became of great value for the industrial and academic synthesis of aromatic compounds, go back to Reppe’s celebrated studies on the use of 1 in synthetic organic chemistry [3].
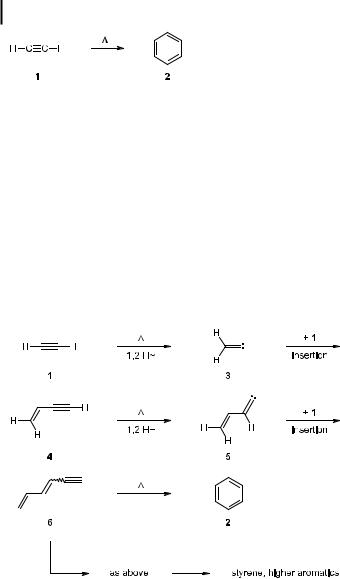
Although the Berthelot benzene synthesis must obviously be a multi-step process, its detailed mechanism surprisingly remains unknown to the present day. Being interested in the actual ring-producing step (see below), several years ago we postulated [4] that the overall process might be initiated by the isomerization of 1 to vinylidene carbene (3), a reaction that has been studied in detail by Brown and co-workers (Scheme 2) [5]:
Scheme 2. Berthelot’s benzene synthesis: a possible mechanism.
In the next step, 3 could insert into one of the CaH bonds of 1 to provide vinylacetylene (4, butenyne), a compound that is indeed generated when 1 is pyrolyzed. Repetition of the carbene formation step could then produce 5, which, by another insertion step, would lead to hexa-1,3-dien-5-yne (6, mixture of isomers). This is already an isomer of benzene, and that it can cyclize to 2 was shown by us many years ago [6]. Obviously, 6 could also be a precursor of styrene and other oligomers of 1. The mechanism proposed in Scheme 2 could also be of importance in connection with soot formation from smaller hydrocarbon fragments, and account for the formation of 2 in interfeller space.

5.2 The Aromatization of Hexa-1,3-dien-5-yne to Benzene: Mechanism and Preparative Applications 171
This chapter first addresses the mechanism and preparative uses of the 6 ! 2 cyclization in modern aromatic chemistry, and then proceeds to the construction of larger aromatic systems (‘‘carbon networks’’ incorporating benzene rings), acetylenes serving as starting materials in both cases. In a third and final section, devoted to the construction of novel bridged aromatics (cyclophanes) from acetylenes, it is shown that substrates containing triple bonds have lost little of their importance and fascination since Berthelot’s days, and that highly topical problems of synthetic aromatic chemistry can be solved starting from alkynes. Clearly, in a chapter for a monograph like the present one, the author’s own contributions to this field of hydrocarbon chemistry will dominate the discussion.
5.2
The Aromatization of Hexa-1,3-dien-5-yne to Benzene: Mechanism and Preparative Applications
Formally, the aromatization of 6 is the dihydro variant of the Bergman cyclization [7]; however, compared to the latter process, the ring-closure of 6 does not require additional (‘‘external’’) hydrogen atoms to proceed. Whereas the mechanism of the Bergman cyclization, involving a benzene-1,4-diyl intermediate, is comparatively clear-cut [8], the aromatization of 6 is more complex and at least three di erent mechanisms are presently discussed for the process (Scheme 3) [9].
Scheme 3. Three routes from hexa-1,3-dien-5-yne (6) to benzene (2).
In alternative (a), ring-formation takes place electrocyclically and leads to isobenzene (7; 1,2,4-cyclohexatriene) as the primary reaction intermediate [10]. This highly strained cycloallene subsequently aromatizes to 2 by hydrogen migration. In the second route, pathway (b), reversible generation of a vinylidene carbene (8; see above) constitutes the first step, and is followed by 1,6-carbon hydrogen insertion. Finally, in pathway (c), vinyl radicals of type 9
1725 From Acetylenes to Aromatics: Novel Routes -- Novel Products
are produced by hydrogen atom addition to the triple bond. Cyclization is again achieved by a pericyclic step, and 2 is ultimately produced by the splitting o of a chain-carrying hydrogen atom. Because of their di erent activation energies [8, 9], these three mechanisms show a di erent response to an increase in temperature. Whereas up to ca. 550 C, the cycloaromatization preferentially follows route (a), beyond this temperature the other alternatives become increasingly competitive. At 625 C, the radical route dominates the aromatization reaction, and at 750 C the process is almost exclusively governed by the carbene mechanism.
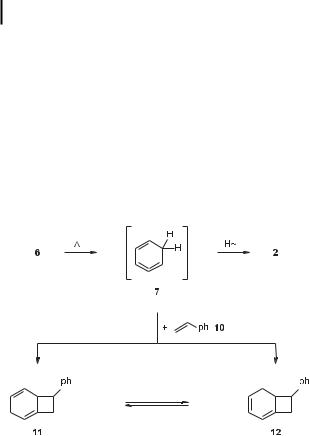
That isobenzene (7) is indeed produced as a reactive intermediate during the 6 ! 2 cyclization was demonstrated by pyrolyzing acetylene in the presence of styrene (10) at 200 C (Scheme 4) [11]:
Scheme 4. The trapping of isobenzene (7).
Besides other homo and hetero dimers and trimers of 6, the trapping products 11 and 12 were obtained, with the conjugated diene 12 presumed to be a secondary product formed by thermal equilibration of the [2þ2] cycloadduct 11 of 7 and 10.
Preparatively important applications of the isobenzene route to aromatics were discovered by thermolysis of the dibenzofulvene derivative 13 (Scheme 5). When this hydrocarbon, which incorporates the hexa-1,3-dien-5-yne unit as a subsystem, is heated in toluene in a sealed ampoule at 350 C, the condensed aromatic hydrocarbon 14 is produced in excellent yield (87 %) [11].
Repeating the cycloaromatization at lower temperatures (250 C, toluene) leads to formation of the highly crowded dispiro compound 15, which is again produced in good yield (74 %) and represents a formal dimer of 13. Taking authentic 15 to the original temperature again provides 14, also in good yield (80 %).
To account for this novel route to condensed aromatic systems, which – as the examples in Scheme 6 illustrate – can apparently be generalized, it has been suggested [9, 11, 12] that the isobenzene derivatives 17 are initially produced from their acetylenic precursors 16 by electrocyclization (Scheme 6).
