
Chen The electron capture detector
.pdf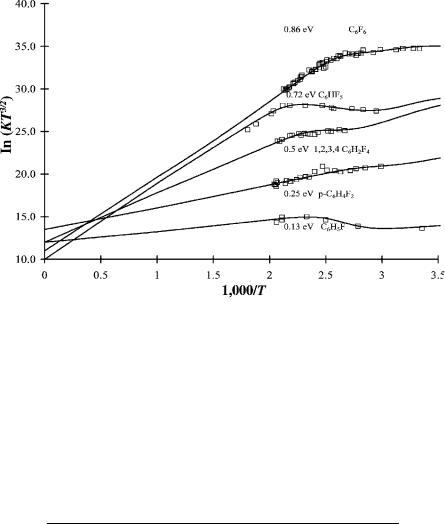
AROMATIC HALIDES |
277 |
Figure 11.5 ECD data as ln KT3/2 versus 1,000/T for fluorobenzenes illustrating multiple states and nondissociative capture, from [34, 35]. The analysis appears in this work. The parameters for the calculated curves are given in Table 11.6.
The electron affinities of several pentafluorophenyl compounds have been determined using the TCT method. Values for C6F5NO2 and C6F5Cl have been measured using the ECD. The increment per fluorine addition ranges from 0.1 eV/F for the nitro compound to 0.16 eV/F for C6F5CN. The largest increment per fluorine atom among the substituted benzene compounds occurs for 1,4-C6F4(CN)2, which is 0.2 eV/F atom [37].
TABLE 11.6 ECD Parameters for Fluorobenzenes
Compound |
ln(A1) (eV) |
E1 (eV) |
Qan |
Ea (eV) |
C6H5F* |
35.2 |
0.63 |
0.80 |
0.13 0.05 |
C6H5F |
35.2 |
0.20 |
0.40 |
0.07 0.05 |
p-C6H4F2 |
35.4 |
0.39 |
0.22 |
0.25 0.05 |
p-C6H4F2* |
35.4 |
0.19 |
1.00 |
0.19 0.05 |
1,2,3,4-C6H2F4* |
35.5 |
0.28 |
0.48 |
0.52 0.05 |
1,2,3,4-C6H2F4 |
35.8 |
0.10 |
0.88 |
0.40 0.05 |
C6HF5* |
34.9 |
0.17 |
0.31 |
0.72 0.05 |
C6HF5 |
35.5 |
0.05 |
1.04 |
0.43 0.05 |
C6F6* |
34.7 |
0.04 |
0.04 |
0.86 0.02 |
C6F6 |
34.9 |
[0.01] |
1.00 |
0.61 0.05 |
* Excited state.
278 THERMAL ELECTRONS AND ENVIRONMENTAL POLLUTANTS
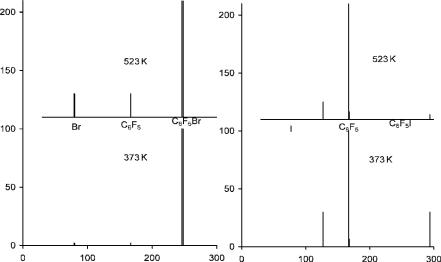
Figure 11.6 Negative-ion mass spectra for C6F5Br and C6F5I, from [39].
The electron affinities of C6Cl6 and C6HCl5 have been determined by TCT [37, 38]. The value for C6Cl6 has also been determined by ECD [23, 35]. The ECD value is about 0.15 eV higher than the TCT value, as is the ECD C6F5Cl value. In the ECD data for C6F5Cl an excited state with a lower electron affinity is observed. By analogy to the fluorobenzenes two states are anticipated. Dissociative electron attachment is not observed in the NIMS spectra taken at 373 K and 523 K. For the majority of these compounds the activation energy is greater than 1 eV, so the only feasible reaction is nondissociative thermal electron attachment. In the case of C6F5Br and C6F5I both dissociative and nondissociative capture is observed. The relative intensities of the C6F5( ), Br( ), or I( ) ions give the electron affinity of C6F5. The relative intensity of Br( ) to the C6F5( ) ions is about 1 while the ratio for the I( ) to the C6F5( ) ions is 0.2. In the spectrum of C6F5I( ) the parent negative ion is observed at 373 K, but is much smaller at 523 K. These data are illustrated in Figure 11.6. The Ea of the C6F5 radical falls between that of I, 3.07 eV, and Br, 3.37 eV or 3.22(10) eV [33, 37, 39, 40].
CURES-EC calculated the Ea for the C6X5 radicals as 3.15, 2.90, 3.05, and 2.90 eV, for X from F to I. Since the Ea of the phenyl radical is 1.10 eV the Ea increments per X are 0.4 eV/F, 0.36 eV/Cl, 0.4 eV/Br, and 0.36 eV/I. The first and second fluorine atoms have a larger increment since the experimental Ea of p-C6H4F is 1.7 eV, while that of C6H3F2 is 2.1 eV. The CURES-EC values are 1.72 eV and 2.15 eV. The last three replacements contribute an average of 0.3 eV/F. The experimental Ea of the mono-chlorophenide radicals range from 1.4(2) eV to 1.7(2) eV, giving increments from 0.3 to 0.6 eV/Cl. The CURES-EC values for o-C6H4Cl and p-C6H4Cl are 1.4 eV and 1.7 eV [37].
AROMATIC HALIDES |
279 |
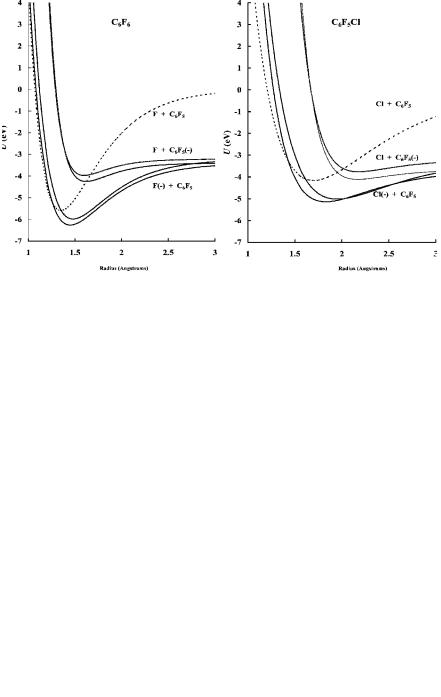
Figure 11.7 Morse potential energy curves for C6F5Cl and C6F6, Eql(2/2) compounds. Calculated from electron impact, ECD, and TCT electron affinities.
With the Ea of the molecules and radicals, bond dissociation energies, and electron impact data, the Morse potential energy curves for the C6F5X compounds can be calculated. They are shown in Figures 11.7 and 11.8. There are two curves dissociating to each of the complementary limits C6F5 þ X( ) and C6F5( ) þ X. The
Figure 11.8 Morse potential energy curves for C6F5Br and C6F5I DEC(2) compounds. Calculated from electron impact and TCT electron affinities.
280 THERMAL ELECTRONS AND ENVIRONMENTAL POLLUTANTS
EDEA for C6F5I, C6F5Br, C6F5Cl, and C6F6 are 0.2, 0.2, 0.5, and 1.2 eV. Dissociative electron attachment occurs in C6F5I and C6F5Br. The two bonding curves are drawn with the experimental Ea for C6F6 and C6F5Cl, while the two other curves are drawn with an assumed bond energy, the VEa, and electron impact ion distributions. The activation energy of about 0.4 eV needed for the reaction to form the ground state of C6F5Cl results from this ‘‘backside crossing.’’ The activation energy to the first excited state is small, but rapid stabilization to the ground state is possible. These curves are both M(2) since the Ea and VEa are positive but the EDEA is negative. Electron attachment will primarily take place via the first excited state, but the ground state will participate at higher temperatures. The two regions are observed in the ECD data for C6F5Cl. The TCT value, 0.82(10) eV, is assigned to the excited state and the higher ECD value, 1.00(10) eV, to the ground state [33–35]. At sufficiently high temperatures or with hyperthermal electrons, dissociative electron attachment will take place. The curves for C6F6 are constructed in a similar manner.
The bonding curves for C6F5I and C6F5Br are qualitative since only one Ea has been measured. In the case of C6F5Br the low-temperature reaction will be dominated by nondissociative capture. At higher temperatures both immediate and sequential dissociation from the first excited state can take place. Because both reactions have the same activation energy, the intensities of the Br( ) and C6F5( ) ions should be the same. For C6F6I the molecular ion is formed at low temperatures via the first excited state. This anion state can also dissociate to yield I( ) and C6F5( ). The activation energy for the formation of I( ) is greater than for C6F5( ), as reflected in the NIMS data. In electron impact studies the parent negative ions of C6F6, C6F5Cl, and C6F5Br were detected at thermal energies, but the parent negative ion of C6F5I was not observed [39, 40].
The electron affinities of the lower chlorobenzenes and several chlorobenzenes substituted by the fluoro and trifluoromethyl groups have been measured using the ECD. These data have been revisited using two states with dissociation that give higher electron affinities [34]. The Ea for the chlorobenzenes range from 0.17 eV to 1.15 eV. This corresponds to an increment in the Ea of 0.2 eV/Cl atom. Figure 11.9 shows the ECD data for the chlorofluorobenzene. Figure 11.10 presents the data for representative mixed halogenated compounds [6, 34]. The ECD parameters given in Table 11.7 are used to calculate the curves in these figures. In these cases the energy for dissociative electron attachment and the bond dissociation energy are obtained from the ECD data. The bond dissociation energies for several aromatic C X bonds determined in this manner are given in Table 11.8. The value for the iodobenzene is an upper limit because dissociative thermal electron attachment is exothermic. The electron affinities for the CF3-substituted compounds calculated using CURES-EC are higher than the experimental values. This could indicate that the ECD values are for excited states, or it might simply mean that the CURES-EC calculations give upper limits. The Ea of C6F5X, where X is NO2, CN, CHO, CO(CH3), and aromatic halogen compounds, are evaluated in the appendices [33, 37].

AROMATIC HALIDES |
281 |
Figure 11.9 ECD data as ln KT3/2 versus 1,000/T for chlorobenzenes illustrating multiple states and dissociative and nondissociative capture, data from [6, 34]. The analysis appears in this work. The parameters for the calculated curves are given in Table 11.7.
Figure 11.10 Plots of ECD data as ln KT3/2 versus 1,000/T for mixed halogenated benzenes. The parameters for the calculated curves are given in Table 11.7.

282 THERMAL ELECTRONS AND ENVIRONMENTAL POLLUTANTS
TABLE 11.7 Kinetic and Thermodynamic Properties of Dissociative
Electron Attachment
|
ln(A1) |
|
|
|
|
ln(A2) (eV) D(C Cl) |
|
Species |
(eV) |
E1 (eV) |
Q |
Ea (eV) |
E2 (eV) |
||
3-Cl-acetophenone |
34.9 |
0.03 |
0.99 |
0.67(5) |
1.05(5) |
28.5(3) |
3.96(5) |
4-Cl-acetophenone |
34.8 |
0.00 |
0.60 |
0.64(5) |
1.05(5) |
29.4(4) |
4.01(5) |
C6H5Cl |
35.1 |
0.07 |
0.38 |
0.17(10) |
0.73(5) |
24.2(3) |
4.09(5) |
p-C6H4Cl2 |
35.2 |
0.03 |
0.45 |
0.29(5) |
0.72(5) |
29.0(3) |
4.03(5) |
m-C6H4Cl2 |
35.1 |
0.04 |
0.81 |
0.30(5) |
0.69(5) |
25.7(3) |
3.97(5) |
o-C6H4Cl2 |
35.1 |
0.03 |
0.70 |
0.30(5) |
0.65(5) |
25.7(3) |
3.92(5) |
s-C6H3Cl3 |
35.6 |
0.04 |
0.64 |
0.48(5) |
0.91(5) |
32.9(3) |
4.04(5) |
s-C6H2Cl4 |
35.8 |
0.03 |
0.94 |
0.69(5) |
1.10(5) |
34.2(3) |
4.02(5) |
1-Cl-naphthalene |
35.6 |
0.14 |
1.01 |
0.34(5) |
0.82(5) |
29.7(2) |
3.94(5) |
|
|
|
|
|
|
|
|
The value in parentheses are uncertainties in the last figure.
The electron affinities of the three mono-chloroanthracenes measured by TCT and ECD agree within the uncertainties. The Ea of bromoanthracene and bromopyrene measured by the collisional ionization techniques could be for excited states based on CURES-EC and the substitution effects. The Ea of 9-bromoanthracene, 0.62 eV, is lower than that of anthracene and lower than the CURES-EC value of 0.95 eV. The evaluated value for 9-chloroanthracene is 0.86 eV. The value for the bromocompound should be higher. The Ea for bromopyrene, 0.75 eV, is larger than that for pyrene but is still lower than the CURES-EC value of 1.1 eV. Many of the
TABLE 11.8 Bond Dissociation Energies (in eV) from ECD Data
Species |
EDEA |
Ea(X) |
D(C X) |
D(Lit) |
C6H5I |
0.10(5) |
3.055 |
<3.15 |
2.85 |
C6H5Br |
0.25(3) |
3.365 |
3.62 |
3.65 |
C6H5Cl |
0.56(3) |
3.615 |
4.18 |
4.15 |
3-Cl-acetophenone |
0.38(3) |
3.615 |
3.99 |
— |
4-Cl-acetophenone |
0.41(3) |
3.615 |
4.02 |
— |
p-C6H4Cl2 |
0.43(3) |
3.615 |
4.05 |
— |
m-C6H4Cl2 |
0.39(3) |
3.615 |
4.02 |
— |
o-C6H4Cl2 |
0.35(3) |
3.615 |
3.97 |
— |
s-C6H3Cl3 |
0.43(3) |
3.615 |
4.05 |
— |
s-C6H2Cl4 |
0.41(3) |
3.615 |
4.03 |
— |
1,4CF3C6H4Cl |
0.41(4) |
3.615 |
4.03 |
— |
1,2CF3C6H4Cl |
0.34(5) |
3.615 |
3.97 |
— |
1,2,4CF3C6H3Cl2 |
0.28(5) |
3.615 |
3.91 |
— |
1,3,4-FC6H3Cl2 |
0.41(5) |
3.615 |
4.03 |
— |
1-Cl-naphthalene |
0.32(3) |
3.615 |
3.94 |
— |
1,3CF3C6H4Br |
0.10(3) |
3.365 |
3.47 |
— |
1,2FC6H4Br |
0.20(3) |
3.365 |
3.57 |
— |
1-Br-naphthalene |
0.06(3) |
3.365 |
3.42 |
— |
|
|
|
|
|

AROMATIC HALIDES |
283 |
TABLE 11.9 Electron Affinities (in eV) of Chlorobenzenes from Reduction Potentials, ECD, and CURES-EC [22 and this work]
Species |
Ea(ER) |
Ea(ECD) |
Ea(CURES-EC) |
C6H5Cl |
0.10 |
0.17(5) |
0.10 |
p-C6H4Cl2 |
0.27 |
0.29(5) |
0.25 |
m-C6H4Cl2 |
0.27 |
0.29(5) |
0.25 |
o-C6H4CCl2 |
0.25 |
0.30(5) |
0.23 |
1,3,5-C6H3Cl3 |
0.49 |
0.48(5) |
0.47 |
1,2,3-C6H3Cl3 |
0.51 |
— |
0.45 |
1,2,4-C6H3Cl3 |
0.47 |
— |
0.49 |
1,2,4,5-C6H2Cl4 |
0.66 |
0.65(5) |
0.71 |
1,2,3,4-C6H2Cl4 |
0.73 |
— |
0.72 |
1,2,3,5-C6H2Cl4 |
0.68 |
— |
0.70 |
C6Cl5H |
0.90 |
0.85(10) |
0.90 |
C6Cl6 |
1.15 |
1.15(5) |
1.15 |
|
|
|
|
TCT values could exist for excited states. Based on this analysis, the AEa should be larger, as indicated in the appendices [41, 42].
11.3.2 Electron Affinities from Reduction Potentials and CURES-EC
The electron affinities of the chlorobenzene isomers have been determined by scaling half-wave reduction potentials [22]. With higher gas phase values higher values are obtained from reduction potentials. These are compared to the ECD and CURES-EC values in Table 11.9. The CURES-EC-calculated values for the above compounds support experimental quantities and suggest that the Ea of all halogenated benzenes can be calculated. The CURES-EC values are listed in Table 11.10. The Ea
TABLE 11.10 Calculated Valence-State Electron Affinities (in eV) of Halobenzenes [this work]
Species |
X F |
X Cl |
X Br |
X I |
C6H6 |
0.756 |
0.756 |
0.756 |
0.756 |
C6H5X |
0.10 |
0.10 |
0.30 |
0.50 |
p-C6H4X2 |
0.19 |
0.25 |
0.53 |
0.76 |
m-C6H4X2 |
0.21 |
0.25 |
0.54 |
0.79 |
o-C6H4CX2 |
0.16 |
0.23 |
0.50 |
0.74 |
1,3,5-C6H3X3 |
0.45 |
0.47 |
0.72 |
1.01 |
1,2,3-C6H3X3 |
0.39 |
0.45 |
0.73 |
0.92 |
1,2,4-C6H3X3 |
0.35 |
0.49 |
0.78 |
0.92 |
1,2,4,5-C6H2X4 |
0.50 |
0.71 |
0.95 |
1.17 |
1,2,3,4-C6H2X4 |
0.55 |
0.72 |
0.95 |
1.20 |
1,2,3,5-C6H2X4 |
0.54 |
0.70 |
0.98 |
1.16 |
C6X5H |
0.72 |
0.90 |
1.24 |
1.50 |
C6X6 |
0.85 |
1.15 |
1.55 |
1.77 |
|
|
|
|
|
284 THERMAL ELECTRONS AND ENVIRONMENTAL POLLUTANTS
for the bromobenzenes range from 0.3 eV to 1.55 eV; those for the iodobenzenes range from 0.5 eV to 1.77 eV. For each substitution the Ea increases by about 0.25 eV. The Ea for the C6X6 compounds are larger than the values for the C6F5X compounds. The differences increase in the order F ¼ 0 to I ¼ 0.36 eV. By comparison with the Morse potential energy curves for C6F5X, the electron impact and NIMS data for these compounds should be similar.
The electron affinities of several chlorinated biphenyls and chlorinated naphthalenes have been determined from half-wave reduction potentials [22]. The electron affinity of 1-Cl naphthalene measured in the ECD has been used to scale the values for the other chloronaphthalens. The solution energy differences were set to the same value, 2.05(5) eV, for compounds with the same number of chlorine atoms up to three chlorines. For four or more chlorines up to the fully chlorinated naphthalene the mddG is 1.95(5) eV. For the fully chlorinated compound the mddG is 1.85(5) eV. This gives for Ea a range of 0.3 to 1.57 eV or 0.2 eV/Cl atom from naphthalene to the fully chlorinated naphthalene. The CURES-EC calculations support these values. They are given in Table 11.11.
The electron affinities of the chlorinated biphenyls are lower than those of the chlorinated napthalenes. The electron affinity of phenylpentachlorobenzene, C12H5Cl5, is about 1.0 eV, while that of C12Cl10 is only 0.3 eV higher. The first five chlorine atoms raise the electron affinity of biphenyl by about 0.8 eV, while the second five only increase the electron affinity by approximately 0.2 eV. The CURES-EC calculations agree with the E1/2 values for substitution on a given ring, but are higher for the substitution on two rings. For example, the decachlorobiphenyl CURES-EC value is 1.5 eV versus the reduction potential value of 1.3 eV. These differences can be seen in the values listed in Table 11.12. The CURES-EC method gives upper limits for these Ea.
11.3.3Negative-Ion Mass Spectra and Electron Affinities
The negative-ion mass spectra of halogenated ethylenes, benzenes, biphenyls, and naphthalenes can be related to experimental electron affinities [39]. The groundstate Ea for C2Cl4 and C2HCl3 are obtained from the temperature dependence of the parent negative-ion intensities. The NIMS data at 373 K and 523 K support these values. The parent negative ions are observed at 373 K, but the relative intensity of the parent negative ion at 523 K is much smaller for C2HCl3 than C2Cl4. Dissociative thermal electron attachment is observed at both temperatures [39].
The mono and dichlorobenzenes do not show parent negative ions at 373 K or 523 K. This does not imply that the electron affinity is negative, but rather that other processes occur. Both dissociative and nondissociative capture are observed for the tri and tetrachlorobenzenes. For the penta and hexachlorobenzenes no dissociative attachment is observed. For the biphenyls with two chlorines ion molecule reactions predominate. With three chlorines, two on one ring and the third on the other ring, molecular ions are observed at 373 K. With four or more chlorines molecular ions are observed at both high and low temperatures, and dissociative electron
|
|
AROMATIC HALIDES |
285 |
||
TABLE 11.11 Electron Affinities of Chloronaphthalenes |
|
||||
from Reduction Potentials and CURES-EC [22 and this work] |
|
||||
|
|
|
|
|
|
Species |
Ea(ER) |
Ea(C-EC) |
mddG |
|
|
|
|
|
|
|
|
1-C10H7Cl |
0.37 |
0.36 |
2.05 |
|
|
2-C10H7Cl |
0.33 |
0.35 |
2.05 |
|
|
1,2-C10H6Cl2 |
0.58 |
0.64 |
2.05 |
|
|
1,3-C10H6Cl2 |
0.56 |
0.52 |
2.05 |
|
|
1,4-C10H6Cl2 |
0.56 |
0.51 |
2.05 |
|
|
1,5-C10H6Cl2 |
0.55 |
0.51 |
2.05 |
|
|
1,6-C10H6Cl2 |
0.51 |
0.50 |
2.05 |
|
|
1,7-C10H6Cl2 |
0.52 |
0.49 |
2.05 |
|
|
1,8-C10H6Cl2 |
0.61 |
0.65 |
2.05 |
|
|
2,3-C10H6Cl2 |
0.54 |
0.50 |
2.05 |
|
|
2,6-C10H6Cl2 |
0.58 |
0.64 |
2.05 |
|
|
2,7-C10H6Cl2 |
0.58 |
0.64 |
2.05 |
|
|
1,2,3-C10H5Cl3 |
0.81 |
0.74 |
2.00 |
|
|
1,2,4-C10H5Cl3 |
0.80 |
0.75 |
2.00 |
|
|
1,2,6-C10H5Cl3 |
0.78 |
0.75 |
2.00 |
|
|
1,2,6-C10H5Cl3 |
0.74 |
0.78 |
2.00 |
|
|
1,2,7-C10H5Cl3 |
0.75 |
0.74 |
2.00 |
|
|
1,2,8-C10H5Cl3 |
0.85 |
0.74 |
2.00 |
|
|
1,3,5-C10H5Cl3 |
0.78 |
0.77 |
2.00 |
|
|
1,3,6-C10H5Cl3 |
0.73 |
0.79 |
2.00 |
|
|
1,3,7-C10H5Cl3 |
0.73 |
0.78 |
2.00 |
|
|
1,3,8-C10H5Cl3 |
0.82 |
0.77 |
2.00 |
|
|
1,4,5-C10H5Cl3 |
0.82 |
0.75 |
2.00 |
|
|
1,4,6-C10H5Cl3 |
0.74 |
0.77 |
2.00 |
|
|
1,6,7-C10H5Cl3 |
0.76 |
0.76 |
2.00 |
|
|
1,2,3,4-C10H4Cl4 |
1.02 |
0.98 |
1.95 |
|
|
1,2,3,5-C10H4Cl4 |
1.00 |
1.00 |
1.95 |
|
|
1,2,3,7-C10H4Cl4 |
0.97 |
0.92 |
1.95 |
|
|
1,2,4,6-C10H4Cl4 |
0.97 |
1.02 |
1.95 |
|
|
1,3,5,7-C10H4Cl4 |
0.97 |
1.03 |
1.95 |
|
|
1,3,5,8-C10H4Cl4 |
1.04 |
1.02 |
1.95 |
|
|
1,4,5,8-C10H4Cl4 |
1.07 |
1.00 |
1.95 |
|
|
1,4,6,7-C10H4Cl4 |
0.99 |
1.02 |
1.95 |
|
|
1,2,3,5,7-C10H3Cl5 |
1.07 |
1.29 |
1.85 |
|
|
C10Cl8 |
1.57 |
1.63 |
1.85 |
|
|
attachment takes place for molecules with up to five or six chlorines. With additional chlorine substitution only the parent negative ion is observed. In the chlorinated biphenyls containing a hydroxyl group, the loss of HCl and Cl2 is observed. In the case of tri and tetra-chlorinated p-terphenyls, parent negative ions are observed at 373 K and 523 K, but the intensity at 523 K is much lower

286 THERMAL ELECTRONS AND ENVIRONMENTAL POLLUTANTS
TABLE 11.12 Electron Affinities (in eV) of Chlorinated Biphenyls from Reduction Potentials and CURES-EC
[22 and this work]
Species |
EaðE1=2Þ |
Ea(CEC) |
Difference |
||
4-C12H9Cl |
0.44 |
0.40 |
0.04 |
||
3-C12H9Cl |
0.39 |
0.41 |
|
0.02 |
|
2-C12H9Cl |
0.40 |
0.38 |
0.02 |
||
2,3-C12H8Cl2 |
0.54 |
0.61 |
|
0.07 |
|
2,4-C12H8Cl2 |
0.52 |
0.66 |
|
0.14 |
|
2,5-C12H8Cl2 |
0.56 |
0.66 |
|
0.10 |
|
3,4-C12H8Cl2 |
0.63 |
0.66 |
|
0.13 |
|
3,5-C12H8Cl2 |
0.60 |
0.65 |
|
0.05 |
|
2,3-C12H8Cl2 |
0.54 |
0.61 |
|
0.07 |
|
2,40-C12H8Cl2 |
0.46 |
0.48 |
|
0.02 |
|
2,20-C12H8Cl2 |
0.37 |
0.60 |
|
0.23 |
|
3,30-C12H8Cl2 |
0.47 |
0.66 |
|
0.19 |
|
4,40-C12H8Cl2 |
0.50 |
0.66 |
|
0.16 |
|
2,3,5-C12H7Cl3 |
0.82 |
0.83 |
|
0.01 |
|
2,3,6-C12H7Cl3 |
0.66 |
0.70 |
|
0.04 |
|
2,4,5-C12H7Cl3 |
0.76 |
0.89 |
|
0.13 |
|
3,4,5-C12H7Cl3 |
0.90 |
0.86 |
0.04 |
||
2,3,4,5-C12H6Cl4 |
1.02 |
1.06 |
|
0.04 |
|
2,3,4,6-C12H6Cl4 |
0.92 |
0.88 |
0.04 |
||
2,3,5,6-C12H6Cl4 |
0.91 |
0.91 |
|
0.00 |
|
3,4,30,40-C12H6Cl4 |
0.94 |
1.05 |
|
0.11 |
|
2,5,20,50-C12H6Cl4 |
0.80 |
0.80 |
|
0.00 |
|
3,5,30,50-C12H6Cl4 |
0.98 |
1.02 |
|
0.04 |
|
2,6,20,60-C12H6Cl4 |
0.58 |
0.61 |
|
0.03 |
|
2,3,4,5,7-C12H5Cl5 |
1.03 |
1.03 |
|
0.00 |
|
20,50,2,4,5-C12H5Cl5 |
0.83 |
0.78 |
0.05 |
||
|
|||||
20,40,50,2,4,5-C12H4Cl6 |
1.04 |
1.16 |
|
0.12 |
|
20,4060,2,4,6-C12H4Cl6 |
0.89 |
0.96 |
|
0.07 |
|
C12Cl10 |
1.39 |
1.57 |
|
0.18 |
|
than at 373 K. At 523 K dissociative electron attachment predominates. The CURES-EC calculated Ea for p-terphenyl is 0.4 eV so the electron affinities for trichlorophenyl should be about 1 eV.
Chloronaphthalene undergoes ion molecule reactions with methane to give the M H ion at 373 K. At 523 K the major ion is chloride, in agreement with the ECD data and a EDEA of 0.5 eV. The parent negative ion for the dichloronaphthalenes is observed at 373 K, indicating an electron affinity greater than 0.4 eV. Dissociative thermal electron attachment is also observed, indicating that the –EDEA is about 0.5 eV. With three or more chlorines we observe only the parent negative ion, showing stabilization to a ground state with a higher Ea.
