
Chen The electron capture detector
.pdf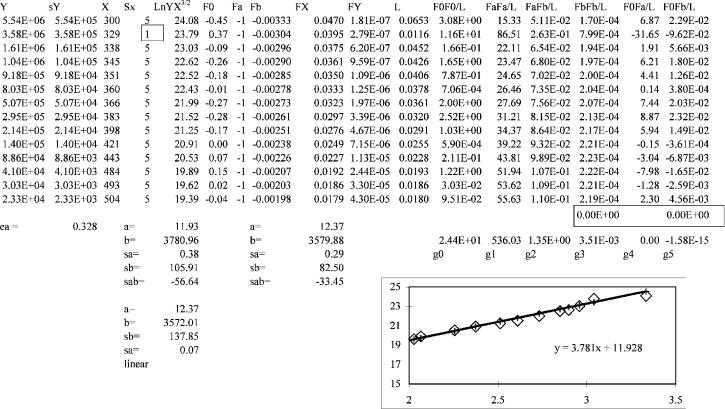
347
Figure AIII.4 The error for one temperature is reduced to 1 K, as opposed to 5 K for the other data point. This illustrates the importance of proper weighting.
348 APPENDIX III
To illustrate the effect of weighting the values, a smaller error in T, 1 instead of 5 , will be assumed for one point. If the second data point had been part of a concentration study where the temperature was stabilized and multiple samples injected, this might be possible. As shown in Figure AIII.4, the values of the slope and intercept are 11.93(18) and 3,781(106), giving an Ea of 0.328(10) and Qan of 1.00(2). This is an example of how important weighting can be and how other data and their uncertainties may be used to improve the value from the data treatment.

 APPENDIX IV
APPENDIX IV
Tables of Evaluated Electron Affinities
TABLE A1.1 |
Atoms (in eV) |
|
|
|
|
|
|
|
|
|
|
|
|
N |
Atom |
AEa |
Uncertainty |
Method |
Reference |
Year |
|
|
|
|
|
|
|
1 |
H |
0.754209 |
3.0E-06 |
calc |
[9] |
1962 |
2 |
He |
0þ |
— |
— |
this work |
2003 |
3 |
Li |
0.618069 |
4.4E-05 |
THD |
[10] |
1996 |
4 |
Be |
0þ |
— |
— |
this work |
2003 |
5 |
B |
0.279723 |
4.4E-05 |
THD |
[11] |
1998 |
6 |
C |
1.262119 |
4.4E-05 |
THD |
[12] |
1998 |
7 |
N |
0þ |
— |
— |
this work |
2003 |
8 |
O |
1.461110 |
7.0E-08 |
THD |
[13] |
1999 |
9 |
F |
3.401290 |
3.0E-06 |
THD |
[14] |
2001 |
10 |
Ne |
0þ |
— |
— |
this work |
2003 |
11 |
Na |
0.547930 |
2.5E-05 |
THD |
[2] |
1985 |
12 |
Mg |
0þ |
— |
— |
this work |
2003 |
13 |
Al |
0.432830 |
5.0E-05 |
THD |
[15] |
1998 |
14 |
Si |
1.389521 |
2.0E-05 |
THD |
[12] |
1998 |
15 |
P |
0.7464 |
4.0E-04 |
THD |
[2] |
1985 |
16 |
S |
2.077103 |
3.0E-06 |
THD |
[16] |
1995 |
17 |
Cl |
3.612740 |
3.0E-05 |
THD |
[17] |
1987 |
18 |
Ar |
0þ |
— |
— |
this work |
2003 |
19 |
K |
0.50147 |
1.2E-04 |
THD |
[18] |
2000 |
20 |
Ca |
0.024546 |
8.7E-05 |
THD |
[19] |
1996 |
21 |
Sc |
0.19 |
2.0E-02 |
PES |
[20] |
1981 |
22 |
Ti |
0.08 |
1.0E-02 |
PES |
[21] |
1987 |
23 |
V |
0.53 |
1.0E-02 |
PES |
[22] |
1998 |
24 |
Cr |
0.676 |
1.2E-04 |
PES |
[22] |
1998 |
25 |
Mn |
0þ |
— |
— |
this work |
2003 |
26 |
Fe |
0.151 |
3.0E-03 |
PES |
[23] |
1986 |
27 |
Co |
0.6633 |
6.1E-04 |
THD |
[24] |
1998 |
28 |
Ni |
1.15716 |
1.3E-04 |
THD |
[24] |
1998 |
29 |
Cu |
1.235792 |
4.4E-04 |
THD |
[25] |
1992 |
30 |
Zn |
0þ |
— |
— |
this work |
2003 |
349
350 |
APPENDIX IV |
|
|
|
|
|
TABLE A1.1 |
(Continued) |
|
|
|
|
|
|
|
|
|
|
|
|
N |
Atom |
AEa |
Uncertainty |
Method |
Reference |
Year |
|
|
|
|
|
|
|
31 |
Ga |
0.41 |
4.0E-02 |
THD |
[26] |
1998 |
32 |
Ge |
1.232712 |
1.5E-04 |
THD |
[12] |
1998 |
33 |
As |
0.814 |
8.0E-03 |
PES |
[27] |
1998 |
34 |
Se |
2.020682 |
4.4E-05 |
THD |
[28] |
1988 |
35 |
Br |
3.363583 |
4.4E-05 |
THD |
[29] |
1989 |
36 |
Kr |
0þ |
— |
— |
this work |
2003 |
37 |
Rb |
0.485920 |
2.0E-05 |
THD |
[30] |
1978 |
38 |
Sr |
0.05206 |
4.4E-05 |
THD |
[31] |
1997 |
39 |
Y |
0.308 |
1.2E-02 |
PES |
[20] |
1981 |
40 |
Zr |
0.427 |
1.4E-02 |
PES |
[2] |
1985 |
41 |
Nb |
0.894 |
2.5E-02 |
PES |
[2] |
1985 |
42 |
Mo |
0.7472 |
2.0E-04 |
PES |
[22] |
1998 |
43 |
Tc |
0.55 |
1.5E-01 |
EST |
[2] |
1985 |
44 |
Ru |
1.04638 |
2.0E-04 |
THD |
[32] |
1999 |
45 |
Rh |
1.142890 |
2.0E-04 |
THD |
[24] |
1998 |
46 |
Pd |
0.56214 |
1.2E-04 |
THD |
[24] |
1998 |
47 |
Ag |
1.30447 |
2.0E-05 |
THD |
[22] |
1998 |
48 |
Cd |
0þ |
— |
— |
this work |
2003 |
49 |
In |
0.404 |
9.0E-03 |
THD |
[33] |
1998 |
50 |
Sn |
1.112067 |
1.5E-04 |
THD |
[12] |
1998 |
51 |
Sb |
1.474020 |
2.0E-05 |
THD |
[34] |
1997 |
52 |
Te |
1.970876 |
7.0E-06 |
THD |
[35] |
1996 |
53 |
I |
3.059000 |
1.0E-05 |
THD |
[36] |
1992 |
54 |
Xe |
0þ |
— |
— |
this work |
2003 |
55 |
Cs |
0.471640 |
6.0E-05 |
THD |
[37] |
1998 |
56 |
Ba |
0.144620 |
6.0E-05 |
THD |
[38] |
1995 |
57 |
La |
0.470 |
2.6E-02 |
THD |
[39] |
1998 |
58 |
Ce |
0.955 |
2.6E-02 |
THD |
[40] |
2002 |
59 |
Pr |
0.962 |
2.6E-02 |
THD |
[41] |
2002 |
60 |
Nd |
0.050 |
— |
EST |
[4] |
1997 |
61 |
Pm |
0þ |
— |
EST |
[4] |
1997 |
62 |
Sm |
0.05 |
— |
EST |
[4] |
1997 |
63 |
Eu |
0.05 |
— |
EST |
[4] |
1997 |
64 |
Gd |
0.10 |
— |
EST |
[4] |
1997 |
65 |
Tb |
0.10 |
— |
EST |
[4] |
1997 |
66 |
Dy |
0.15 |
— |
EST |
[4] |
1997 |
67 |
Ho |
0þ |
— |
EST |
[4] |
1997 |
68 |
Er |
0þ |
— |
EST |
[4] |
1997 |
69 |
Tm |
1.029 |
2.2E-02 |
THD |
[42] |
2002 |
70 |
Yb |
0.01 |
— |
EST |
[4] |
1997 |
71 |
Lu |
0.34 |
— |
THD |
[43] |
2001 |
72 |
Hf |
0.10 |
— |
EST |
[4] |
1997 |
73 |
Ta |
0.323 |
1.2E-02 |
PES |
[2] |
1985 |
74 |
W |
0.815 |
4.0E-03 |
PES |
[44] |
1992 |
75 |
Re |
0.150 |
1.0E-01 |
SI |
[45] |
1970 |
76 |
Os |
1.0778 |
1.5E-04 |
THD |
[46] |
2000 |
|
|
|
TABLES OF EVALUATED ELECTRON AFFINITIES |
351 |
||
TABLE A1.1 |
(Continued) |
|
|
|
|
|
|
|
|
|
|
|
|
N |
Atom |
AEa |
Uncertainty |
Method |
Reference |
Year |
|
|
|
|
|
|
|
77 |
Ir |
1.5644 |
1.5E-04 |
THD |
[47] |
1999 |
78 |
Pt |
2.12510 |
5.0E-05 |
THD |
[47] |
1999 |
79 |
Au |
2.30863 |
3.0E-05 |
THD |
[2] |
1985 |
80 |
Hg |
0þ |
— |
EST |
this work |
2003 |
81 |
Tl |
0.377 |
1.3E-02 |
PD |
[48] |
2000 |
82 |
Pb |
1.10 |
5.0E-02 |
PD |
[49] |
1973 |
83 |
Bi |
0.942362 |
1.3E-05 |
PES |
[50] |
2001 |
84 |
Po |
1.9 |
3.0E-01 |
EST |
this work |
2003 |
85 |
At |
2.8 |
2.0E-01 |
EST |
this work |
2003 |
86 |
Rn |
0þ |
— |
EST |
this work |
2003 |
87 |
Fr |
0.460 |
— |
EST |
this work |
2003 |
88 |
Ra |
0.170 |
— |
EST |
this work |
2003 |
89 |
Ac |
0þ |
— |
EST |
this work |
2003 |
90 |
Th |
0.05 |
— |
EST |
this work |
2003 |
91 |
Pa |
0.05 |
— |
EST |
this work |
2003 |
92 |
U |
0.05 |
— |
EST |
this work |
2003 |
93 |
Np |
0þ |
— |
EST |
this work |
2003 |
94 |
Pu |
0.05 |
— |
EST |
this work |
2003 |
|
|
|||||
TABLE A1.2 Main Group Homonuclear Diatomic Molecules (in eV) |
|
|||||
|
|
|
|
|
|
|
AN |
Mol |
AEa |
Uncertainty |
Method |
Reference |
Year |
|
|
|
|
|
|
|
3 |
Li2 |
0.509 |
0.009 |
PES |
[51] |
1994 |
5 |
B2 |
1.300 |
0.400 |
PES |
[52] |
1993 |
6 |
C2 |
3.269 |
0.006 |
M |
[6] |
2003 |
8 |
O2 |
1.070 |
0.100 |
M |
this work |
2003 |
9 |
F2 |
3.080 |
0.050 |
M |
[6] |
2003 |
11 |
Na2 |
0.430 |
0.015 |
PES |
[53] |
1989 |
13 |
Al2 |
1.460 |
0.060 |
PES |
[54] |
1998 |
14 |
Si2 |
2.200 |
0.010 |
PES |
[55] |
1993 |
15 |
P2 |
0.610 |
0.025 |
PES |
[56] |
1985 |
16 |
S2 |
1.690 |
0.015 |
M |
[6] |
2003 |
17 |
Cl2 |
2.450 |
0.020 |
M |
Table 9.1 |
2003 |
19 |
K2 |
0.497 |
0.015 |
PES |
[53] |
1989 |
20 |
Ca2 |
0.025 |
— |
EST |
this work |
2003 |
29 |
Cu2 |
0.840 |
0.010 |
PES |
[57] |
1990 |
31 |
Ga2 |
1.600 |
0.100 |
PES |
[58] |
1994 |
32 |
Ge2 |
2.074 |
0.001 |
PES |
[59] |
1995 |
33 |
As2 |
0.739 |
0.001 |
PES |
[60] |
1998 |
34 |
Se2 |
1.940 |
0.070 |
PES |
[61] |
1989 |
35 |
Br2 |
2.560 |
0.020 |
M |
Table 9.1 |
2003 |
37 |
Rb2 |
0.498 |
0.015 |
LPD |
[53] |
1989 |
38 |
Sr2 |
0.052 |
— |
EST |
this work |
2003 |
47 |
Ag2 |
1.100 |
0.008 |
PES |
[57] |
1990 |
49 |
In2 |
1.270 |
0.100 |
PES |
[62] |
1990 |
352 |
APPENDIX IV |
|
|
|
|
|
TABLE A1.2 |
(Continued) |
|
|
|
|
|
|
|
|
|
|
|
|
AN |
Mol |
AEa |
Uncertainty |
Method |
Reference |
Year |
|
|
|
|
|
|
|
50 |
Sn2 |
1.965 |
0.010 |
PES |
[63] |
1999 |
51 |
Sb2 |
1.282 |
0.008 |
PES |
[64] |
1992 |
52 |
Te2 |
1.920 |
0.070 |
PES |
[65] |
1992 |
53 |
I2 |
2.524 |
0.005 |
PES |
[66] |
1997 |
55 |
Cs2 |
0.469 |
0.015 |
PES |
[53] |
1989 |
56 |
Ba2 |
0.145 |
— |
EST |
this work |
2003 |
79 |
Au2 |
1.940 |
0.001 |
PES |
[57] |
1990 |
81 |
Tl2 |
0.950 |
0.100 |
PES |
[62] |
1990 |
82 |
Pb2 |
1.366 |
0.008 |
PES |
[65] |
1992 |
83 |
Bi2 |
1.271 |
0.008 |
PES |
[67] |
1991 |
The electron affinities of the Group II homonuclear diatomic molecules should be greater than the electron affinity of the atom.
REFERENCES
1.Chen, E. C. M. and Wentworth, W. E. J. Chem. Educ. 1975, 52 486.
2.Hotop, H. and Lineberger, W. C. J. Phys. Chem. Ref. Data 1985, 14, 731.
3.Wheeler, J. C. J. Chem. Educ. 1997, 74, 123.
4.Nadeau, M-J.; Garwan, M. A.; Zhao, X-L.; and Litherland, A. E. Nuclear Instr. Meth. Phys. Res. B 1997 123, 521.
5.Andersen, T.; Haugen, H. K.; and Hotop, H. J. Phys. Chem. Ref. Data 1999, 28, 1511.
6.National Institute of Standards and Technology (NIST). Chemistry WebBook, 2003. Available at http://webbook.nist.gov.
7.Rienstra-Kiracofe, J. C.; Tschumper, G. S.; Schaefer, H. F.; Nandi, S.; and Ellison, G. B.
Chem. Rev. 2002, 102, 231.
8.Sheer, M. D. J. Res. Nat. Bur. Stds. 1970, A74, 37.
9.Pekeris, C. L. Phys. Rev. 1962, 126, 1470.
10.Haeffler, G.; Hanstorp, G.; Kiyan, I.; Klinkmiller, A. E.; Ljungblad, U.; and Pegg, D. J. Phys. Rev. A 1996, 53, 4127.
11.Scheer, M.; Bilodeau, R. C.; and Haugen, H. K. Phys. Rev. Lett. 1998, 80, 2562–2565.
12.Scheer, M.; Bilodeau, R. C.; Brodie, C. A.; and Haugen, H. K. Phys. Rev. A 1998, 58, 2844.
13.Valli, C.; Blondel, C.; and Delsart, C. Phys. Rev. A 1999, 59, 3809.
14.Blondel, C.; Delsart, C.; and Goldfarb, F. J. Phys. B—Atom. Molec. Opt. Phys., 2001, 34, L281.
15.15 Scheer, M.; Bilodeau, R. C.; Thogresen, J.; and Haugen, H. K. Phys. Rev. A 1998, 57, R1493.
16.Blondel, C. Phys. Scr. 1995, T58, 31.
17.Trainham, R.; Fletcher, G. D.; and Larson, D. J. J. Phys. B 1987, 20, L777.
TABLES OF EVALUATED ELECTRON AFFINITIES |
353 |
18.Andersson, K. T.; Sandstrom, J.; Kiyan, I. Y.; Hanstorp, D.; and Pegg, D. J. Phys. Rev. A 2000, 62, 22503.
19.Petrunin, V. V.; Andersen, H. H.; Balling, P.; and Andersen, T. Phys. Rev. Lett. 1996, 76, 744.
20.Feigerle, C. S.; Herman, Z.; and Lineberger, C. W. J. Electron. Spectrosc. Relat. Phenom. 1981, 23, 441.
21.Ilin, R. N.; Sakharov, V. I.; and Serenkov, I. T. Opt. Spectros. (USSR), 1987, 62, 578.
22.Bilodeau, R. C.; Scheer, M.; and Haugen, H. K. J. Phys. B 1998, 31, 3885.
23.Leopold, D. G. and Lineberger, W. C. J. Chem. Phys. 1986, 85, 51.
24.Scheer, M.; Brodie, C. A.; Bilodeau, R. C.; and Haugen, H. K. Phys. Rev. A 1998, 58, 2051.
25.Taylor, K. J.; Pettiettehall, C. L.; Cheshnovsky, O.; and Smalley, R. E. J. Chem. Phys. 1992, 96, 3319.
26.Williams, W. W.; Carpenter, D. L.; Covington, A. M.; Koepnick, M. C.; Calabrese, D.; and Thompson, J. S. J. Phys. B 1998, 31, L341–L345.
27.Lippa, T. P.; Xu, S. J.; Lyapustina, S. A.; Nilles, J. M.; and Bowen, K. H. J. Chem. Phys. 1998, 109, 10727.
28.Mansour, N. B.; Edge, C. J.; and Larson, D. J. Nucl. Instrum. Meth. Phys. Res. B 1988, 31, 313.
29.Blondel, C.; Cacciani, P.; Delsart, C.; and Trainham, R. Phys. Rev. A 1989, 40, 3698.
30.Frey, P.; Breyer, F.; and Hotop, H. J. Phys. B: At. Mol. Opt. Phys. 1978, 11, L589.
31.Andersen, H. H.; Petrunin, V. V.; Kristensen, P.; and Andersen, T. Phys. Rev. A 1997, 55, 3247.
32.Norquist, P. L.; Beck, D. R.; Bilodeau, R. C.; Scheer, M.; Srawley, R. A.; and Haugen, H. K. Phys. Rev. A 1999, 59, 1896–1902.
33.Williams, W. W.; Carpenter, D. L.; Covington, A. M.; Thompson, J. S.; Kvale, T. J.; and Seely, D. G. Phys. Rev. A 1998, 58, 3582.
34.Scheer, M.; Haugen, H. K.; and Beck, D. R. Phys. Rev. Lett. 1997, 79, 4104.
35.Haeffler, G.; Klinkmu¨ller, A. E.; Rangell, J.; Berzinsh, U.; and Hanstorp, D. Z. Phys. D 1996, 38, 211.
36.Hanstorp, D. and Gustafsson, M. J. Phys. B 1992, 25, 1773.
37.Scheer, M.; Thogersen, J.; Bilodeau, R. C.; Brodie, C. A.; and Haugen, H. K. Phys. Rev. Lett. 1998, 80, 684.
38.Petrunin, V. V.; Volstad, J. D.; Balling, P.; Kristensen, K.; and Andersen, T. Phys. Rev. Lett. 1995, 75, 1911.
39.Covington, A. M.; Calabrese, D.; Thompson, J. S.; and Kvale, T. J. J. Phys. B 1998, 31, L855.
40.Davis, V. T. and Thompson, J. S. Phys. Rev. Lett. 2002, 88, 073003.
41.Davis, V. T. and Thompson, J. S. J. Phys. B 2002, 35, L11.
42.Davis, V. T. and Thompson, J. S. Phys. Rev. A 2002, 6501, 0501.
354 APPENDIX IV
43.Davis, V. T. and Thompson, J. S. J. Phys. B 2001, 354, L433.
44.Bengali, A. A.; Casey, S. M.; Cheng, C-L.; Dick, J. P.; Fenn, T.; Villaalta, P. W.; and Leopold, D. G. J. Amer. Chem. Soc. 1992, 114, 5257.
45.Sheer, M. D. J. Res. Nat. Bur. Stds. 1970, 74A, 37.
46.Bilodeau, R. C.; and Haugen, H. K. Phys. Rev. Lett. 2000, 85, 534–537.
47.Bilodeau, R. C.; Scheer, M.; Haugen, H. K.; and Brooks, R. L. Phys. Rev. A 1999, 61, 12505.
48.Carpenter, D. L.; Covington, A. M.; and Thompson J. S. Phys. Rev. A 2000, 61, 42501.
49.Feldman, D. In Proc. 8th Intern. Conf. on Electr. Atom. Collis, Belgrade, 1973.
50.Bilodeau, R. C. and Haugen, H. K. Phys. Rev. A 2001, 6402, 4501.
51.Sarkas, H. W.; Arnold, S. T.; Hendricks, J. H.; Slager, V. L.; and Bowen, K. H. Z. Phys. D 1994, 29, 209.
52.Reid, C. J. Int. J. Mass Spectrom. Ion Proc. 1993, 127, 147.
53.McHugh, K. M.; Eaton, J. G.; Lee, G. H.; Sarkas, H. W.; Kidder, L. H.; Snodgrass, J. T.; Manaa, M. R.; and Bowen, K. H. J. Chem. Phys. 1989, 91, 3792.
54.Li, X.; Wu, H.; Wang, X.; and Wang, L. Phys. Rev. Lett. 1998, 81, 1909.
55.Arnold, C. C.; Kitsopoulos, T. N.; and Neumark, D. M. J. Chem. Phys. 1993, 99, 766.
56.Snodgrass, J. T.; Coe, J. V.; Freidhoff, C. B.; McHugh, K. M.; and Bowen, K. H. Chem. Phys. Lett. 1985, 122, 352.
57.Ho, J.; Ervin, K. M.; and Lineberger, W. C. J. Chem. Phys. 1990, 93, 6987.
58.Cha, C.-Y.; Gantefo¨r, G.; and Eberhardt, W. J. Chem. Phys. 1994, 100, 995.
59.Arnold, C. C.; Xu, C. S.; Burton, G. R.; and Neumark, D. M. J. Chem. Phys. 1995, 102, 6982.
60.Lippa, T. P.; Xu, S. J.; Lyapustina, S. A.; Nilles, J. M.; and Bowen, K. H. J. Chem. Phys. 1998, 109, 10727.
61.Snodgrass, J. T.; Coe, J. V.; McHugh, K. M.; Freidhoff, C. B.; and Bowen, K. H. J. Phys. Chem. 1989, 93, 1249.
62.Gausa, M.; Gantefo¨r, G.; Lutz, H. O.; and Meiwes-Broer, K-H. Int. J. Mass Spectrom. Ion Proc. 1990, 102, 227.
63.Moravec, V. D.; Klopcic, S. A.; and Jarrold, C. C. J. Chem. Phys. 1999, 110, 5079– 5088.
64.Polak, M. L.; Gerber, G.; Ho, J.; and Lineberger, W. C. J. Chem. Phys. 1992, 97, 8990.
65.Ho, J.; Polak, M. L.; and Lineberger, W. C. J. Chem. Phys. 1992, 96, 144.
66.Zanni, M. T.; Taylor, T. R.; Greenblatt, B. J.; Miller, W. H.; and Neumark, D. M. J. Chem. Phys. 1997, 107, 7613.
67.Polak, M. L.; Ho, J.; Gerber, G.; Lineberger, W. C. J. Chem. Phys. 1991, 95, 3053.

TABLES OF EVALUATED ELECTRON AFFINITIES |
355 |
TABLE A2.1 CH Molecules by Value (in eV)
EVAL |
NIST |
Name/Formula |
MW |
Mtd. |
Reference |
|
|
|
|
|
|
0.07(2) |
0.048 |
Benzene, 1,2,4,5-tetramethyl- (C10H14) |
134 |
E |
[2] |
0.10(5) |
— |
Styrene (C8H8) |
104 |
E |
[2] |
0.10(5) |
0.108 |
Benzene, 1,2,3,5-tetramethyl- (C10H14) |
134 |
E |
[2] |
0.12(5) |
0.121 |
Benzene, hexamethyl- (C12H18) |
162 |
E |
[2] |
0.13(5) |
0.130 |
Biphenyl (C12H10) |
154 |
E |
[2] |
0.14(5) |
0.143 |
Naphthalene, 2-methyl- (C11H10) |
142 |
E |
[2] |
0.16(2) |
0.200 |
Naphthalene (C10H8) |
128 |
E |
[1] |
0.16(5) |
0.160 |
Naphthalene, 1-methyl- (C11H10) |
142 |
E |
[2] |
0.16(5) |
0.147 |
Naphthalene, 1-ethyl- (C12H12) |
156 |
E |
[2] |
0.16(5) |
0.160 |
Naphthalene, 2,6-dimethyl- (C12H12) |
156 |
E |
[2] |
0.16(5) |
0.156 |
Diphenylmethane (C13H12) |
168 |
E |
[2] |
0.17(5) |
0.173 |
Indene (C9H8) |
116 |
E |
[2] |
0.17(5) |
0.173 |
Naphthalene, 2,3-dimethyl- (C12H12) |
156 |
E |
[2] |
0.18(5) |
0.182 |
Benzene, pentamethyl- (C11H16) |
148 |
E |
[2] |
0.19(5) |
0.195 |
Naphthalene, 2-ethyl- (C12H12) |
156 |
E |
[2] |
0.21(5) |
0.247 |
Naphthalene, 1,4-dimethyl- (C12H12) |
156 |
E |
[2] |
0.28(5) |
0.278 |
Fluorene (C13H10) |
166 |
E |
[2] |
0.29(2) |
0.285 |
Triphenylene (C18H12) |
228 |
E |
[1] |
0.30(2) |
0.307 |
Phenanthrene (C14H10) |
178 |
E |
[1] |
0.32(5) |
0.321 |
Diphenylethyne (C14H10) |
178 |
E |
[2] |
0.39(5) |
0.390 |
(E)-stilbene (C14H12) |
180 |
E |
[2] |
0.39(5) |
0.390 |
Ethylene, 1,1-diphenyl- (C14H12) |
180 |
E |
[2] |
0.40(5) |
0.890 |
Biphenylene (C12H8) |
152 |
CI |
[3] |
0.42(4) |
0.397 |
Chrysene (C18H12) |
228 |
E |
[1] |
0.54(3) |
0.542 |
Picene (C22H14) |
278 |
E |
[1] |
0.55(3) |
0.534 |
Benzo[e]pyrene (C20H12) |
252 |
E |
[1] |
0.58(1) |
0.545 |
Benzo[c]phenanthrene (C18H12) |
228 |
E |
[1] |
0.60(5) |
0.550 |
Anthracene, 1-methyl- (C15H12) |
192 |
CI |
[3] |
0.61(2) |
0.500 |
Pyrene (C16H10) |
202 |
E |
[1] |
0.67(3) |
0.591 |
Dibenz[a,j]anthracene (C22H14) |
278 |
E |
[1] |
0.68(2) |
0.530 |
Anthracene (C14H10) |
178 |
E, P, T |
[1, 4, 5] |
0.69(3) |
0.595 |
Dibenz[a,h]anthracene (C22H14) |
278 |
E |
[1] |
0.69(3) |
— |
Dibenz[a,c]anthracene (C22H14) |
278 |
E |
[1] |
0.72(1) |
0.390 |
Benz[a]anthracene (C18H12) |
228 |
E |
[1] |
0.80(10) |
0.550 |
1,3,5,7-Cyclooctatetrene (C8H8) |
104 |
E, P, T |
[1, 6–8] |
0.80(5) |
0.403 |
Acenaphthylene (C12H8) |
152 |
E |
[1] |
0.80(5) |
0.470 |
Coronene (C24H12) |
300 |
P, CI |
[9, 10] |
0.82(4) |
0.815 |
Benzo[a]pyrene (C20H12) |
252 |
E, T |
[1] |
0.82(5) |
0.630 |
Fluoranthene (C16H10) |
202 |
E |
[1] |
0.84(5) |
0.694 |
Azulene (C10H8) |
128 |
E, P, T |
[1, 11, 12] |
0.89(5) |
0.420 |
Benzo[ghi]perylene (C22H12) |
276 |
CI |
[9] |
0.97(1) |
0.973 |
Perylene (C20H12) |
252 |
E, P, T |
[1, 5, 13] |
1.00(20) |
1.000 |
Dibenzo[a,g]corannulene radical |
350 |
CI |
[9] |
|
|
(C28H14) |
|
|
|
1.08(4) |
1.067 |
Naphthacene (C18H12) |
228 |
E, P, T |
[1, 5, 14] |
1.16(20) |
1.160 |
Diindenochrysene radical (C26H12) |
324 |
CI |
[9] |
1.39(5) |
1.392 |
Pentacene (C22H14) |
278 |
T |
[5] |
|
|
|
|
|
|
356 |
APPENDIX IV |
|
|
|
|
TABLE A2.2 CH Molecules by Molecular Weight (in eV) |
|
|
|
||
|
|
|
|
|
|
EVAL |
NIST |
Name/Formula |
MW |
Mtd. |
Reference |
|
|
|
|
|
|
0.10(5) |
— |
Styrene(C8H8) |
104 |
E |
[2] |
0.80(10) |
0.550 |
1,3,5,7-Cyclooctatetrene (C8H8) |
104 |
E, P, T |
[1, 6–8] |
0.17(5) |
0.173 |
Indene (C9H8) |
116 |
E |
[2] |
0.16(2) |
0.200 |
Naphthalene (C10H8) |
128 |
E |
[1] |
0.84(5) |
0.694 |
Azulene (C10H8) |
128 |
E, P, T |
[1, 11, 12] |
0.07(2) |
0.048 |
Benzene, 1,2,4,5-tetramethyl- (C10H14) |
134 |
E |
[2] |
0.10(5) |
0.108 |
Benzene, 1,2,3,5-tetramethyl- (C10H14) |
134 |
E |
[2] |
0.14(5) |
0.143 |
Naphthalene, 2-methyl- (C11H10) |
142 |
E |
[2] |
0.16(5) |
0.160 |
Naphthalene, 1-methyl- (C11H10) |
142 |
E |
[2] |
0.18(5) |
0.182 |
Benzene, pentamethyl- (C11H16) |
148 |
E |
[2] |
0.40(5) |
0.890 |
Biphenylene (C12H8) |
152 |
CI |
[3] |
0.80(5) |
0.403 |
Acenaphthylene (C12H8) |
152 |
E |
[1] |
0.13(5) |
0.130 |
Biphenyl (C12H10) |
154 |
E |
[2] |
0.16(5) |
0.147 |
Naphthalene, 1-ethyl- (C12H12) |
156 |
E |
[2] |
0.16(5) |
0.160 |
Naphthalene, 2,6-dimethyl- (C12H12) |
156 |
E |
[2] |
0.17(5) |
0.173 |
Naphthalene, 2,3-dimethyl- (C12H12) |
156 |
E |
[2] |
0.19(5) |
0.195 |
Naphthalene, 2-ethyl- (C12H12) |
156 |
E |
[2] |
0.21(5) |
0.247 |
Naphthalene, 1,4-dimethyl- (C12H12) |
156 |
E |
[2] |
0.12(5) |
0.121 |
Benzene, hexamethyl- (C12H18) |
162 |
E |
[2] |
0.28(5) |
0.278 |
Fluorene (C13H10) |
166 |
E |
[2] |
0.16(5) |
0.156 |
Diphenylmethane (C13H12) |
168 |
E |
[2] |
0.30(2) |
0.307 |
Phenanthrene (C14H10) |
178 |
E |
[1] |
0.32(5) |
0.321 |
Diphenylethyne (C14H10) |
178 |
E |
[2] |
0.68(2) |
0.530 |
Anthracene (C14H10) |
178 |
E, P, T |
[1, 4, 5] |
0.39(5) |
0.390 |
(E)-stilbene (C14H12) |
180 |
E |
[2] |
0.39(5) |
0.390 |
Ethylene, 1,1-diphenyl- (C14H12) |
180 |
E |
[2] |
0.60(5) |
0.550 |
Anthracene, 1-methyl- (C15H12) |
192 |
CI |
[3] |
0.61(2) |
0.500 |
Pyrene (C16H10) |
202 |
E |
[1] |
0.82(5) |
0.630 |
Fluoranthene (C16H10) |
202 |
E |
[1] |
0.29(2) |
0.285 |
Triphenylene (C18H12) |
228 |
E |
[1] |
0.42(4) |
0.397 |
Chrysene (C18H12) |
228 |
E |
[1] |
0.58(1) |
0.545 |
Benzo[c]phenanthrene (C18H12) |
228 |
E |
[1] |
0.72(1) |
0.390 |
Benz[a]anthracene (C18H12) |
228 |
E |
[1] |
1.08(4) |
1.067 |
Naphthacene (C18H12) |
228 |
E, P, T |
[1, 5, 14] |
0.55(3) |
0.534 |
Benzo[e]pyrene (C20H12) |
252 |
E |
[1] |
0.82(4) |
0.815 |
Benzo[a]pyrene (C20H12) |
252 |
E, T |
[1] |
0.97(1) |
0.973 |
Perylene (C20H12) |
252 |
E, P, T |
[1, 5, 13] |
0.89(5) |
0.420 |
Benzo[ghi]perylene (C22H12) |
276 |
CI |
[9] |
0.54(3) |
0.542 |
Picene (C22H14) |
278 |
E |
[1] |
0.67(3) |
0.591 |
Dibenz[a,j]anthracene (C22H14) |
278 |
E |
[1] |
0.69(3) |
0.595 |
Dibenz[a,h]anthracene (C22H14) |
278 |
E |
[1] |
0.69(3) |
— |
Dibenz[a,c]anthracene (C22H14) |
278 |
E |
[1] |
1.39(5) |
1.392 |
Pentacene (C22H14) |
278 |
T |
[5] |
0.80(5) |
0.470 |
Coronene (C24H12) |
300 |
P, CI |
[9, 10] |
1.00(20) |
1.000 |
Dibenzo[a,g]corannulene (C28H14) |
350 |
CI |
[9] |
1.16(20) |
1.160 |
Diindenochrysene radical (C26H12) |
324 |
CI |
[9] |
|
|
|
|
|
|
