
Garrett R.H., Grisham C.M. - Biochemistry (1999)(2nd ed.)(en)
.pdf3.1 ● Basic Thermodynamic Concepts |
61 |
energy or enthalpy (that is, the number of microscopic states at a given temperature, pressure, and amount of material), then the entropy is given by
S k ln W |
(3.5) |
where k is Boltzmann’s constant (k 1.38 10 23 J/K). This definition is useful for statistical calculations (it is in fact a foundation of statistical thermodynamics), but a more common form relates entropy to the heat transferred in a process:
dSreversible |
dq |
(3.6) |
|
T |
|||
|
|
where dSreversible is the entropy change of the system in a reversible2 process, q is the heat transferred, and T is the temperature at which the heat transfer occurs.
The Third Law: Why Is “Absolute Zero” So Important?
The third law of thermodynamics states that the entropy of any crystalline, perfectly ordered substance must approach zero as the temperature approaches 0 K, and at T 0 K entropy is exactly zero. Based on this, it is possible to establish a quantitative, absolute entropy scale for any substance as
S T CPd ln T |
(3.7) |
0 |
|
where CP is the heat capacity at constant pressure. The heat capacity of any substance is the amount of heat one mole of it can store as the temperature of that substance is raised by one degree. For a constant pressure process, this is described mathematically as
CP |
dH |
(3.8) |
|
dT |
|||
|
|
If the heat capacity can be evaluated at all temperatures between 0 K and the temperature of interest, an absolute entropy can be calculated. For biological processes, entropy changes are more useful than absolute entropies. The entropy change for a process can be calculated if the enthalpy change and free energy change are known.
Free Energy: A Hypothetical but Useful Device
An important question for chemists, and particularly for biochemists, is, “Will the reaction proceed in the direction written?” J. Willard Gibbs, one of the founders of thermodynamics, realized that the answer to this question lay in a comparison of the enthalpy change and the entropy change for a reaction at a given temperature. The Gibbs free energy, G, is defined as
G H TS |
(3.9) |
For any process A 34 B at constant pressure and temperature, the free energy change is given by
G H T S |
(3.10) |
2A reversible process is one that can be reversed by an infinitesimal modification of a variable.

62 Chapter 3 ● Thermodynamics of Biological Systems
If G is equal to 0, the process is at equilibrium, and there is no net flow either in the forward or reverse direction. When G 0, S H/T, and the enthalpic and entropic changes are exactly balanced. Any process with a nonzero G proceeds spontaneously to a final state of lower free energy. If G is negative, the process proceeds spontaneously in the direction written. If G is positive, the reaction or process proceeds spontaneously in the reverse direction. (The sign and value of G do not allow us to determine how fast the process will go.) If the process has a negative G, it is said to be exergonic, whereas processes with positive G values are endergonic.
The Standard-State Free Energy Change
The free energy change, G, for any reaction depends upon the nature of the reactants and products, but it is also affected by the conditions of the reaction, including temperature, pressure, pH, and the concentrations of the reactants and products. As explained earlier, it is useful to define a standard state for such processes. If the free energy change for a reaction is sensitive to solution conditions, what is the particular significance of the standard-state free energy change? To answer this question, consider a reaction between two reactants A and B to produce the products C and D.
A B 34 C D |
(3.11) |
The free energy change for non–standard-state concentrations is given by
G G° RT ln |
[C][D] |
|
(3.12) |
|
[A][B] |
||||
|
|
|||
At equilibrium, G 0 and [C][D]/[A][B] Keq. We then have |
|
|||
G ° RT ln Keq |
(3.13) |
|||
or, in base 10 logarithms, |
|
|||
G ° 2.3RT log10 Keq |
(3.14) |
|||
This can be rearranged to |
|
|||
Keq 10 G°/2.3RT |
(3.15) |
|||
In any of these forms, this relationship allows the standard-state free energy change for any process to be determined if the equilibrium constant is known. More importantly, it states that the equilibrium established for a reaction in solution is a function of the standard-state free energy change for the process. That is, G° is another way of writing an equilibrium constant.
EXAMPLE
The equilibrium constants determined by Brandts at several temperatures for the denaturation of chymotrypsinogen (see previous Example) can be used to calculate the free energy changes for the denaturation process. For example, the equilibrium constant at 54.5°C is 0.27, so
G° (8.314 J/mol K)(327.5 K) ln (0.27)
G° (2.72 kJ/mol) ln (0.27)
G ° 3.56 kJ/mol
The positive sign of G ° means that the unfolding process is unfavorable; that is, the stable form of the protein at 54.5°C is the folded form. On the other hand, the relatively small magnitude of G ° means that the folded form is only slightly favored. Figure 3.4 shows the dependence of G° on temperature for
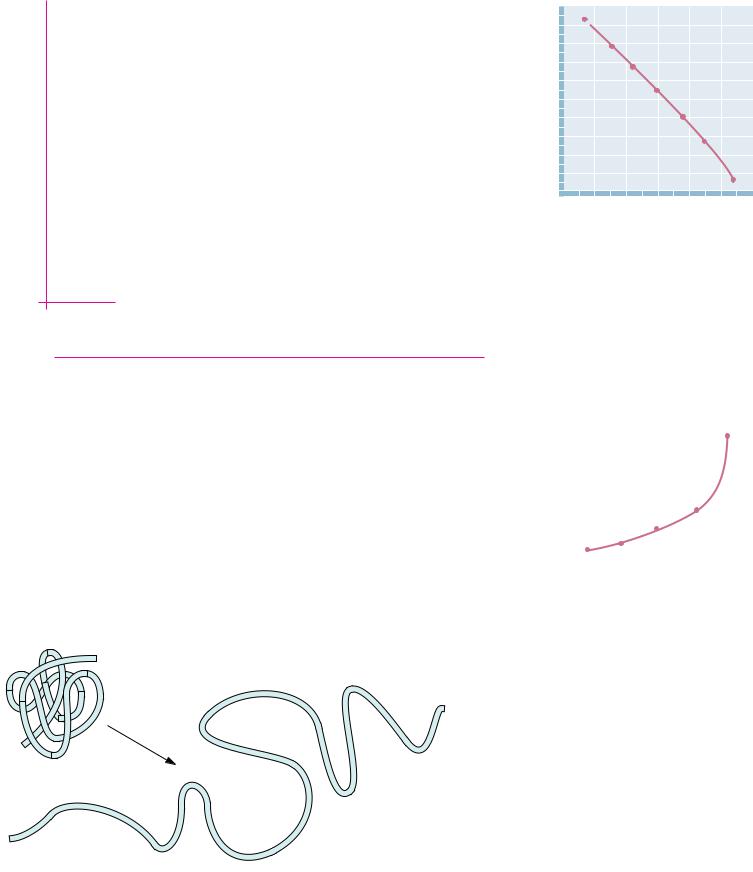
3.2 ● The Physical Significance of Thermodynamic Properties |
63 |
the denaturation data at pH 3 (from the data given in the Example on page 59).
Having calculated both H° and G ° for the denaturation of chymotrypsinogen, we can also calculate S°, using Equation (3.10):
S° |
( G° H°) |
(3.16) |
|
T
At 54.5°C (327.5 K),
S° (3560 533,000 J/mol)/327.5 K
S° 1,620 J/mol K
Figure 3.5 presents the dependence of S° on temperature for chymotrypsinogen denaturation at pH 3. A positive S° indicates that the protein solution has become more disordered as the protein unfolds. Comparison of the value of 1.62 kJ/mol K with the values of S° in Table 3.1 shows that the present value (for chymotrypsinogen at 54.5°C) is quite large. The physical significance of the thermodynamic parameters for the unfolding of chymotrypsinogen becomes clear in the next section.
∆ G, kJ/mol
50 |
52 |
54 |
56 |
58 |
60 |
62 |
|
|
Temperature, °C |
|
|
||
● The dependence of G° on temperature for the denaturation of chymotrypsinogen.
thermodynamics of protein denaturation. I. The denaturation of
chymotrypsinogen. Journal of the American Chemical
Society 86:4291–4301.)
3.2 ● The Physical Significance of Thermodynamic Properties
What can thermodynamic parameters tell us about biochemical events? The best answer to this question is that a single parameter ( H or S, for example) is not very meaningful. A positive H° for the unfolding of a protein might reflect either the breaking of hydrogen bonds within the protein or the exposure of hydrophobic groups to water (Figure 3.6). However, comparison of several thermodynamic parameters can provide meaningful insights about a process. For example, the transfer of Na and Cl ions from the gas phase to aqueous solution involves a very large negative H° (thus a very favorable stabilization of the ions) and a comparatively small S° (Table 3.2). The negative entropy term reflects the ordering of water molecules in the hydration shells of the Na and Cl ions. This unfavorable effect is more than offset by the large heat of hydration, which makes the hydration of ions a very favorable process overall. The negative entropy change for the dissociation of acetic acid in water also reflects the ordering of water molecules in the ion hydration shells. In this case, however, the enthalpy change is much smaller in magnitude. As a result, G° for
Folded
Unfolded
|
2.4 |
|
|
|
|
|
|
|
2.3 |
|
|
|
2.2 |
|
|
•K |
2.1 |
|
|
2.0 |
|
|
|
kJ/molS, |
|
|
|
1.8 |
|
|
|
|
1.9 |
|
|
∆ |
1.7 |
|
|
|
|
|
|
|
1.6 |
|
|
|
1.5 |
|
|
|
1.4 |
|
|
52 |
54 |
56 |
58 |
60 |
|
|
Temperature, °C |
|
|
FIGURE 3.5 ● The dependence of S° on temperature for the denaturation of chymotrypsinogen. (Adapted from Brandts, J. F., 1964. The thermodynamics of protein denaturation. I. The denatura-
tion of chymotrypsinogen. Journal of the American Chemical Society 86:4291– 4301.)
FIGURE 3.6 ● Unfolding of a soluble protein exposes significant numbers of nonpolar groups to water, forcing order on the solvent and resulting in a negative S° for the unfolding process. Yellow spheres represent nonpolar groups; blue spheres are polar and/or charged groups.

64 Chapter 3 ● Thermodynamics of Biological Systems
Table 3.2
Thermodynamic Parameters for Several Simple Processes*
|
H ° |
S ° |
G ° |
CP |
Process |
kJ/mol |
kJ/mol K |
kJ/mol |
kJ/mol K |
|
|
|
|
|
Hydration of ions† |
|
|
|
|
Na (g) Cl (g) 88n Na (aq) Cl (aq) |
760.0 |
0.185 |
705.0 |
|
Dissociation of ions in solution‡ |
|
|
|
|
H2O CH3COOH 88n H3O CH3COO |
0 10.3 |
0.126 |
000027.26 |
0.143 |
Transfer of hydrocarbon from pure liquid to water‡ |
|
|
|
|
Toluene (in pure toluene) 88n toluene (aqueous) |
1.72 |
0.071 |
00022.7 |
000.265 |
*All data collected for 25°C.
†Berry, R. S., Rice, S. A., and Ross, J., 1980. Physical Chemistry. New York: John Wiley.
‡Tanford, C., 1980. The Hydrophobic Effect. New York: John Wiley.
dissociation of acetic acid in water is positive, and acetic acid is thus a weak (largely undissociated) acid.
The transfer of a nonpolar hydrocarbon molecule from its pure liquid to water is an appropriate model for the exposure of protein hydrophobic groups to solvent when a protein unfolds. The transfer of toluene from liquid toluene to water involves a negative S°, a positive G°, and a H° that is small compared to G° (a pattern similar to that observed for the dissociation of acetic acid). What distinguishes these two very different processes is the change in heat capacity (Table 3.2). A positive heat capacity change for a process indicates that the molecules have acquired new ways to move (and thus to store heat energy). A negative CP means that the process has resulted in less freedom of motion for the molecules involved. CP is negative for the dissociation of acetic acid and positive for the transfer of toluene to water. The explanation is that polar and nonpolar molecules both induce organization of nearby water molecules, but in different ways. The water molecules near a nonpolar solute are organized but labile. Hydrogen bonds formed by water molecules near nonpolar solutes rearrange more rapidly than the hydrogen bonds of pure water. On the other hand, the hydrogen bonds formed between water molecules near an ion are less labile (rearrange more slowly) than they would be in pure water. This means that CP should be negative for the dissociation of ions in solution, as observed for acetic acid (Table 3.2).
3.3 ● The Effect of pH on Standard-State Free Energies
For biochemical reactions in which hydrogen ions (H ) are consumed or produced, the usual definition of the standard state is awkward. Standard state for the H ion is 1 M, which corresponds to pH 0. At this pH, nearly all enzymes would be denatured, and biological reactions could not occur. It makes more sense to use free energies and equilibrium constants determined at pH 7. Biochemists have thus adopted a modified standard state, designated with prime ( ) symbols, as in G° , K eq, H° , and so on. For values determined in this way, a standard state of 10 7 M H and unit activity (1 M for solutions, 1 atm for gases and pure solids defined as unit activity) for all other components (in the ionic forms that exist at pH 7) is assumed. The two standard states can be related easily. For a reaction in which H is produced,
A 88n B H |
(3.17) |

3.5 ● |
The Importance of Coupled Processes in Living Things |
65 |
the relation of the equilibrium constants for the two standard states is |
|
|
K eq Keq[H ] |
(3.18) |
|
and G ° is given by |
|
|
G° G° RT ln [H ]
For a reaction in which H is consumed,
A H 88n B
the equilibrium constants are related by
Keq
Keq [H ]
and G° is given by
G° G° RT ln [H1 ] G° RT ln [H ]
3.4 ● The Important Effect of Concentration on
Net Free Energy Changes
(3.19)
(3.20)
(3.21)
(3.22)
Equation (3.12) shows that the free energy change for a reaction can be very different from the standard-state value if the concentrations of reactants and products differ significantly from unit activity (1 M for solutions). The effects can often be dramatic. Consider the hydrolysis of phosphocreatine:
Phosphocreatine H2O 88n creatine Pi |
(3.23) |
This reaction is strongly exergonic and G° at 37°C is 42.8 kJ/mol. Physiological concentrations of phosphocreatine, creatine, and inorganic phosphate are normally between 1 mM and 10 mM. Assuming 1 mM concentrations and using Equation (3.12), the G for the hydrolysis of phosphocreatine is
G 42.8 kJ/mol (8.314 J/mol K)(310 K) ln |
[0.001][0.001] |
|
(3.24) |
[0.001] |
|||
G 60.5 kJ/mol |
|
|
(3.25) |
At 37°C, the difference between standard-state and 1 mM concentrations for such a reaction is thus approximately 17.7 kJ/mol.
3.5 ● The Importance of Coupled Processes in Living Things
Many of the reactions necessary to keep cells and organisms alive must run against their thermodynamic potential, that is, in the direction of positive G. Among these are the synthesis of adenosine triphosphate and other high-energy molecules and the creation of ion gradients in all mammalian cells. These processes are driven in the thermodynamically unfavorable direction via coupling with highly favorable processes. Many such coupled processes are discussed later in this text. They are crucially important in intermediary metabolism, oxidative phosphorylation, and membrane transport, as we shall see.
We can predict whether pairs of coupled reactions will proceed spontaneously by simply summing the free energy changes for each reaction. For example, consider the reaction from glycolysis (discussed in Chapter 19)
66 Chapter 3 ● Thermodynamics of Biological Systems
COO- |
COO- |
||||||
|
|
|
ADP + Pi |
ATP |
|
||
|
2- |
|
|
|
|
||
C |
|
OPO3 |
C |
|
O |
||
|
|
|
|
|
|
|
|
CH2 |
CH3 |
||||||
PEP |
Pyruvate |
||||||
FIGURE 3.7 ● The pyruvate kinase reaction.
involving the conversion of phospho(enol)pyruvate (PEP) to pyruvate (Figure 3.7). The hydrolysis of PEP is energetically very favorable, and it is used to drive phosphorylation of ADP to form ATP, a process that is energetically unfavorable. Using values of G that would be typical for a human erythrocyte:
PEP H2O 88n pyruvate Pi |
G 78 kJ/mol |
(3.26) |
ADP Pi 88n ATP H2O |
G 55 kJ/mol |
(3.27) |
PEP ADP 88n pyruvate ATP |
Total G 23 kJ/mol |
(3.28) |
The net reaction catalyzed by this enzyme depends upon coupling between the two reactions shown in Equations (3.26) and (3.27) to produce the net reaction shown in Equation (3.28) with a net negative G° . Many other examples of coupled reactions are considered in our discussions of intermediary metabolism (Part III). In addition, many of the complex biochemical systems discussed in the later chapters of this text involve reactions and processes with positive G° values that are driven forward by coupling to reactions with a negative G° .
3.6 ● The High-Energy Biomolecules
Virtually all life on earth depends on energy from the sun. Among life forms, there is a hierarchy of energetics: certain organisms capture solar energy directly, whereas others derive their energy from this group in subsequent processes. Organisms that absorb light energy directly are called phototrophic organisms. These organisms store solar energy in the form of various organic molecules. Organisms that feed on these latter molecules, releasing the stored energy in a series of oxidative reactions, are called chemotrophic organisms. Despite these differences, both types of organisms share common mechanisms for generating a useful form of chemical energy. Once captured in chemical form, energy can be released in controlled exergonic reactions to drive a variety of life processes (which require energy). A small family of universal biomolecules mediates the flow of energy from exergonic reactions to the energyrequiring processes of life. These molecules are the reduced coenzymes and the high-energy phosphate compounds. Phosphate compounds are considered high energy if they exhibit large negative free energies of hydrolysis (that is, if G° is more negative than 25 kJ/mol).
Table 3.3 lists the most important members of the high-energy phosphate compounds. Such molecules include phosphoric anhydrides (ATP, ADP), an enol phosphate (PEP), an acyl phosphate (acetyl phosphate), and a guanidino phosphate
(creatine phosphate). Also included are thioesters, such as acetyl-CoA, which do not contain phosphorus, but which have a high free energy of hydrolysis. As noted earlier in this chapter, the exact amount of chemical free energy available from the hydrolysis of such compounds depends on concentration, pH, temperature, and so on, but the G° values for hydrolysis of these substances are substantially more negative than for most other metabolic species. Two important points: first, high-energy phosphate compounds are not long-term energy storage substances. They are transient forms of stored energy, meant to carry energy from point to point, from one enzyme system to another, in the minute-to-minute existence of the cell. (As we shall see in subsequent chapters, other molecules bear the responsibility for long-term storage of energy
3.6 ● The High-Energy Biomolecules |
67 |
Table 3.3
Free Energies of Hydrolysis of Some High-Energy Compounds*
|
G ° |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compound (and Hydrolysis Product) |
(kJ/mol) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Structure |
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phosphoenolpyruvate (pyruvate Pi) |
62.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
–2O3P |
|
|
|
|
O |
|
|
|
|
|
O– |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
C |
|
|
|
|
|
C |
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
N |
|
|
|
|
|
||||||||
3 ,5 -Cyclic adenosine monophosphate (5 -AMP) |
50.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5' |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
H |
|
|
H |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
3' |
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
||||||||||
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
OH |
|
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
–2O3P |
|
|
|
|
|
|
|
|
|
|
CH2 |
|
|
|
|
|
OH |
|
|
|
|
|
O |
|
PO32– |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
1,3-Bisphosphoglycerate (3-phosphoglycerate Pi) |
49.6 |
|
|
|
|
O |
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
C |
|
|
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
–2O3P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
Creatine phosphate (creatine Pi) |
43.3 |
|
|
|
|
|
|
|
|
NHCNCH2COO– |
|
|
|
|
|
||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+NH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
Acetyl phosphate (acetate Pi) |
43.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPO32– |
|
|
|
|||||||||||
Adenosine-5 -triphosphate (ADP Pi) |
35.7† |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
C |
|
|
|
|
NH2 |
N |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
N |
N |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
–O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
P |
|
|
O |
|
|
|
P |
|
|
O |
|
|
|
|
P |
|
|
|
|
|
O |
|
CH |
|
O |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
H |
|
||||||||
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
O |
|
|
|
|
|
|
H |
|
H |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH OH |
|
|||
Adenosine-5 -triphosphate (ADP Pi), excess Mg2 |
30.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
N |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
O– |
O– |
|
|
|
|
|
|
|
|
N |
|
N |
|
||||||||||||||||||||||
Adenosine-5 -diphosphate (AMP Pi) |
35.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
–O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
P |
|
|
O |
|
|
P |
|
|
|
|
O |
|
|
|
|
|
CH |
|
O |
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
H |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
H |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
OH |
|
||
(continued)
68 Chapter 3 ● Thermodynamics of Biological Systems
Table 3.3
Continued
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
G ° |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Compound (and Hydrolysis Product) |
|
|
|
|
|
|
|
|
|
|
|
|
(kJ/mol) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Structure |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Pyrophosphate (Pi Pi) in 5 mM Mg2 |
|
|
|
|
|
|
33.6 |
|
|
|
|
|
|
|
|
|
|
|
–O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P |
|
|
O |
|
|
|
|
P |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Adenosine-5 -triphosphate (AMP PPi), excess Mg2 |
|
|
|
32.3 |
|
|
(See ATP structure on previous page) |
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HN |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
O |
|
H |
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
O– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Uridine diphosphoglucose (UDP glucose) |
|
|
|
|
|
|
31.9 |
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
P |
|
|
|
O |
|
|
|
|
|
P |
|
|
|
O |
|
|
|
|
CH2 O |
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
H |
H |
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
H |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
OH |
|||
Acetyl-coenzyme A (acetate CoA) |
|
|
|
|
|
|
|
|
|
|
|
|
31.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
H3C |
|
OH |
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|||||||||||||||||||||
CH2 |
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
P |
|
|
O |
|
P |
|
O |
|
CH2 |
|
C |
|
CH |
|
C |
|
NH |
|
CH2 |
|
CH2 |
|
C |
|
NH |
|
|
|
CH2 |
|
|
|
|
|
CH2 |
|
|
|
S |
|
|
|
C |
|
|
CH3 |
|
|
|
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
O– |
O– |
H3C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
O |
Adenine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
H |
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
O |
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2 |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
N |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
S-adenosylmethionine (methionine adenosine) |
|
|
|
|
|
|
25.6‡ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
N |
|
N |
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
–OOCCHCH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH |
|
|
|
|
|
S |
|
|
|
|
CH |
|
|
|
|
O |
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
2 |
|
|
|
+ |
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH3+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
H |
H |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
OH |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
supplies.) Second, the term high-energy compound should not be construed to imply that these molecules are unstable and hydrolyze or decompose unpredictably. ATP, for example, is quite a stable molecule. A substantial activation energy must be delivered to ATP to hydrolyze the terminal, or , phosphate group. In fact, as shown in Figure 3.8, the activation energy that must be absorbed by the molecule to break the OOP bond is normally 200 to 400 kJ/mol, which is substantially larger than the net 30.5 kJ/mol released in the hydrolysis reaction. Biochemists are much more concerned with the net release of 30.5 kJ/mol

|
3.6 ● The High-Energy Biomolecules |
69 |
|
|
|
|
|
|
|
|
G ° |
|
|
|
Compound (and Hydrolysis Product) |
(kJ/mol) |
Structure |
|
|
|
|
|
|
|
|
|
|
|
Lower-Energy Phosphate Compounds
Glucose-1-P (glucose Pi) |
21.0 |
Fructose-1-P (fructose Pi) |
16.0 |
Glucose-6-P (glucose Pi) |
13.9 |
sn-Glycerol-3-P (glycerol Pi) |
9.2 |
Adenosine-5 -monophosphate (adenosine Pi) |
9.2 |
HOCH2 O OH
H HO
H CH2 O PO32– OH H
HOCH2 O OH
H HO
H CH2 O PO32–
|
|
|
|
|
|
|
|
|
|
OH H |
|
|
|
|
|
|
|
|
||||
–2O3P |
|
|
|
O |
|
|
|
|
CH2 |
O |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
H |
|
|
|
|
|
H |
|||||||||||
|
|
|
|
|
|
|
H |
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
OH |
H |
|
|
|
||||||
|
|
|
HO |
OH |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
H |
OH |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|||||
–2O3P |
|
|
O |
|
|
CH2 |
|
|
|
|
|
|
|
|||||||||
|
|
C |
|
CH2OH |
||||||||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
NH2 |
|
|
N |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
O– |
|
|
|
|
|
N |
|
|
|
|
|
N |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
–O P |
|
|
|
O |
|
|
|
CH |
O |
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
H |
|
|
|
|||
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
H |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
OH |
|||||||
*Adapted primarily from Handbook of Biochemistry and Molecular Biology, 1976, 3rd ed. In Physical and Chemical Data,
G. Fasman, ed., Vol. 1, pp. 296–304. Boca Raton, FL: CRC Press.
†From Gwynn, R. W., and Veech, R. L., 1973. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. Journal of Biological Chemistry 248:6966–6972.
‡From Mudd, H., and Mann, J., 1963. Activation of methionine for transmethylation. Journal of Biological Chemistry 238:2164–2170.
than with the activation energy for the reaction (because suitable enzymes cope with the latter). The net release of large quantities of free energy distinguishes the high-energy phosphoric anhydrides from their “low-energy” ester cousins, such as glycerol-3-phosphate (Table 3.3). The next section provides a quantitative framework for understanding these comparisons.
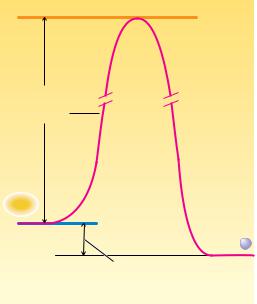
70 Chapter 3 ● Thermodynamics of Biological Systems
Transition state
Activation energy
kJ200–400 mol
ATP
Reactants
ADP + P
Phosphoryl group Products transfer potential
–30.5 kJ/mol
● The activation energies for phosphoryl group-transfer reactions (200 to 400 kJ/mol) are substantially larger than the free energy of hydrolysis of ATP ( 30.5 kJ/mol).
ATP Is an Intermediate Energy-Shuttle Molecule
One last point about Table 3.3 deserves mention. Given the central importance of ATP as a high-energy phosphate in biology, students are sometimes surprised to find that ATP holds an intermediate place in the rank of high-energy phosphates. PEP, cyclic AMP, 1,3-BPG, phosphocreatine, acetyl phosphate, and pyrophosphate all exhibit higher values of G° . This is not a biological anomaly. ATP is uniquely situated between the very high energy phosphates synthesized in the breakdown of fuel molecules and the numerous lower-energy acceptor molecules that are phosphorylated in the course of further metabolic reactions. ADP can accept both phosphates and energy from the higher-energy phosphates, and the ATP thus formed can donate both phosphates and energy to the lower-energy molecules of metabolism. The ATP/ADP pair is an intermediately placed acceptor/donor system among high-energy phosphates. In this context, ATP functions as a very versatile but intermediate energyshuttle device that interacts with many different energy-coupling enzymes of metabolism.
Group Transfer Potential
Many reactions in biochemistry involve the transfer of a functional group from a donor molecule to a specific receptor molecule or to water. The concept of group transfer potential explains the tendency for such reactions to occur. Biochemists define the group transfer potential as the free energy change that occurs upon hydrolysis, that is, upon transfer of the particular group to water. This concept and its terminology are preferable to the more qualitative notion of high-energy bonds.
The concept of group transfer potential is not particularly novel. Other kinds of transfer (of hydrogen ions and electrons, for example) are commonly
