
Astruc D. - Modern arene chemistry (2002)(en)
.pdf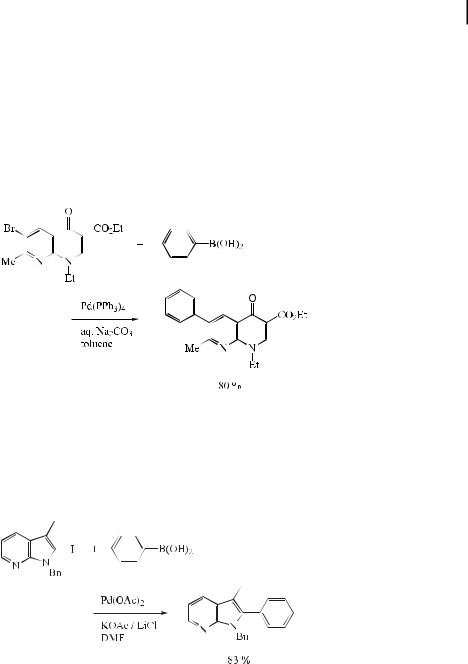
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 73
Since the introduction in 1963 of nalidixic acid (N-ethyl-7-methyl-4-oxo-1,4-dihydro[1,8]- naphthyridinyl-3-carboxylic acid) as a systemic Gram-negative antibacterial agent, many related derivatives have been synthesized. These types of compounds have received much attention and interest by virtue of their chemical and clinical properties. The methods reported in the chemical literature for preparing C-6- and C-3-substituted 4-oxo[1.8]naphthyridines are somewhat limited. These methods involve the condensation of substituted 2- aminopyridines with suitable 3-ethoxyacrylates, followed by cyclization. Recently, such substituted 4-oxo-1,4-dihydro[1.8]naphthyridines have been obtained using the Suzuki reaction (Eq. (38)) [69].
ð38Þ
Palladium-catalyzed functionalization at the 2-positions of various 5- and 7-azaindoles has been performed by the Suzuki reaction. The 2-substituted azaindoles were obtained in moderate to high yields by using Pd(OAc)2, LiCl, and KOAc in DMF at 110 C (Eq. (39)) [70].
ð39Þ
Starting from the commercially available 3,6-dichloropyridazine, 3-amino-6-arylpyridazines, which show acetylcholine esterase inhibiting activities, were prepared in good yields under mild conditions by means of a Suzuki coupling (Eq. (40)) [71].
74 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð40Þ
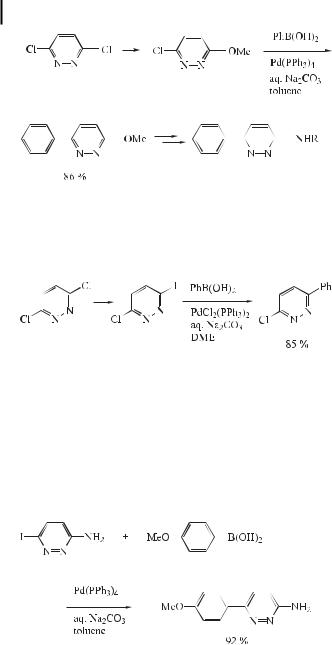
3-Chloro-6-arylpyridazines can also be synthesized in good yields by Suzuki reaction of arylboronic acids with 3-chloro-6-iodopyridazine, the latter being readily accessible from 3,6- dichloropyridazine (Eq. (41)) [72].
ð41Þ
The 3-aminopyridazine skeleton has proved to be interesting from a pharmacological point of view. An interesting group of 3-aminopyridazines are 3-amino-6-(hetero)arylpyridazines as they are intermediates in the synthesis of several pharmacologically active compounds. 6-(Hetero)arylimidazo[1,2-b]pyridazines, for instance, have been proposed for the treatment of anxiety and dementia of the Alzheimer type. The 3-amino-6-(hetero)arylpyridazines have hitherto been prepared by a multistep sequence starting from 3(2H)-pyridazinones. Recently, a new approach towards the synthesis of 3-amino-6-(hetero)arylpyridazines based on the palladium-catalyzed cross-coupling reaction has been proposed (Eq. (42)) [73].
ð42Þ
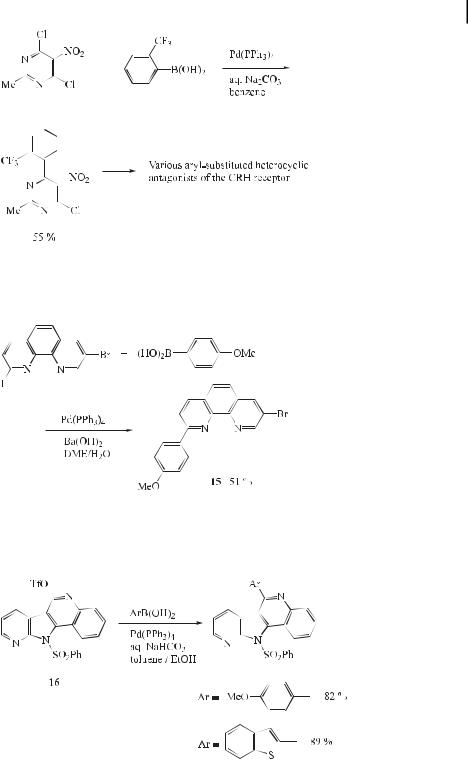
Based on a new substantial body of pharmacological and clinical evidence, it is anticipated that the modulation of the e ects of corticotropin releasing hormone or factor (CRH or CRF) may eventually play a role in the treatment of depression or anxiety-related disorders. This interest in CRH as a new important target for drug discovery is clearly evident. Recently, Gilligan et al. [74] have observed that the Suzuki reaction can be used to synthesize a variety of aryl-substituted heterocyclic antagonists of the CRH receptor (Eq. (43)).
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 75
ð43Þ
Because of the di erent reactivities of iodo and bromo substituents in the Suzuki reaction, selective coupling can be realized. For example, 8-bromo-2-(4-methoxyphenyl)-1,10- phenanthroline (15) can be prepared by reacting the 4-boronic acid derivative of anisole with bromo(iodo)phenanthroline under Suzuki conditions (Eq. (44)) [75].
ð44Þ
Suzuki coupling reactions of 16 with 4-methoxyphenylboronic acid and 2-benzo[b]thiophene- 2-boronic acid in the presence of Pd(PPh3)4 and aqueous NaHCO3 as base, in a mixture of toluene/ethanol (5:1) at 90 C for 2 h, a orded the corresponding coupling products in high yields (Eq. (45)) [76].
ð45Þ
763 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
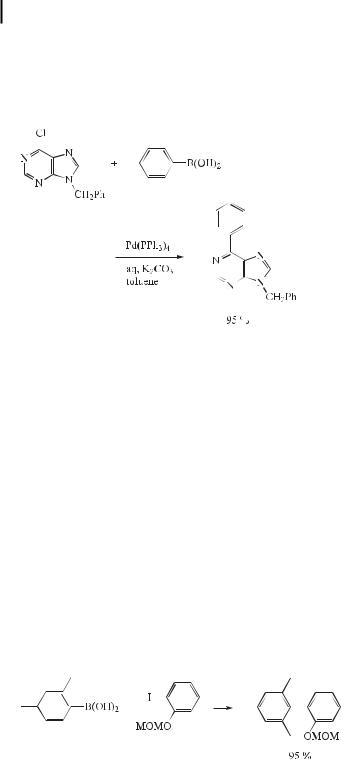
Many 6-alkylaminopurine nucleosides are important adenosine receptor antagonists, and acyclic nucleotide analogues derived from 6-dialkylaminopurines are strong antivirals, antineoplastic agents, and immunomodulators. Recently, several 6-(arylalkynyl)-, 6-(arylalkenyl)-, and 6-(arylalkyl)purines have been reported to exhibit cytokinine activity. Suzuki crosscoupling reactions of 9-benzyl-6-chloropurine with boronic acids have recently been reported to provide 6-substituted purines in moderate to excellent yields (Eq. (46)) [77].
ð46Þ
3.2.3
Coupling of Arylboron Compounds Bearing Sterically Bulky or Electron-Withdrawing Substituents
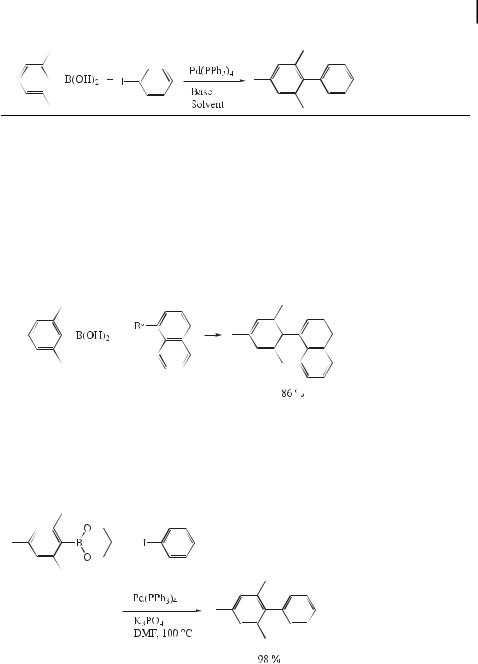
Although steric hindrance of aryl halides is not a major factor in the formation of substituted biaryls, low yields are obtained when ortho-disubstituted arylboronic acids are used. For example, the reaction with mesitylboronic acid proceeds only slowly because of steric hindrance during transmetalation to the palladium(II) complex.
The reaction of mesitylboronic acid with iodobenzene at 80 C in the presence of Pd(PPh3)4 and various bases has been reported [78]. The results are summarized in Table 2.
Aqueous Na2CO3 in benzene or DME (dimethoxyethane) is not e ective as a base for the coupling of mesitylboronic acid, and the reaction does not reach completion even after 2 days. Although side reactions such as homocoupling occur to a negligible extent, the formation of mesitylene as a result of hydrolytic deboronation increases with increasing reaction time. It is noteworthy that such hydrolytic deboronation is faster in benzene/H2O than under the modified conditions using aqueous DME. On the other hand, the addition of stronger bases, such as aqueous NaOH or Ba(OH)2, both in benzene and DME, has a remarkable accelerating e ect on the rate of coupling. By using aqueous Ba(OH)2 in DME at 80 C, mesitylboronic acid couples with iodobenzene within 4 h to give the corresponding biaryl in quantitative yield.
Further coupling reactions of this type are depicted in Eqs. (47) and (48).
ð47Þ
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 77
Tab. 2. Reaction of mesitylboronic acid with iodobenzene under di erent conditions.
Base |
Solvent |
Temp/ C |
Yield/%a |
|
|
|
|
|
|
Time |
8 h |
24 h |
48 h |
|
|
|
|
|
|
|
Na2CO3 |
Benzene/H2O |
80 |
|
25(6) |
77(12) |
84(25) |
Na2CO3 |
DME/H2O |
80 |
|
50(1) |
66(2) |
83(7) |
K3PO4 |
DME/H2O |
80 |
|
70(0) |
|
|
NaOH |
DME/H2O |
80 |
|
95(2) |
|
|
Ba(OH)2 |
DME/H2O |
80 |
|
99(2) |
|
|
a GLC yields of the coupling product based on iodobenzene and the yields of mesitylene are shown in the parentheses.
ð48Þ
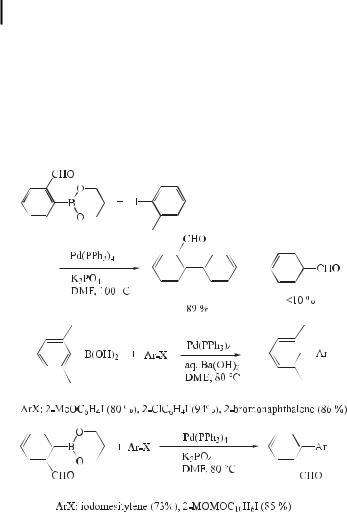
For such sterically hindered boronic acids, an alternative procedure using the esters of boronic acids and an anhydrous base has been developed. Thus, the coupling can readily be achieved by employing the trimethylene glycol ester of mesitylboronic acid and Cs2CO3 or K3PO4 in DMF at 100 C, whereby the coupling product is obtained quantitatively (Eq. (49)) [78].
ð49Þ
Even if there is no great steric hindrance, the reaction under aqueous conditions is often accompanied by undesirable competitive hydrolytic deboronation [79]. Kinetic studies [80] on the reaction of substituted arylboronic acids indicated that electron-withdrawing substituents accelerate the deboronation. Although there is no great di erence between metaand para-substituted phenylboronic acids, substituents at the ortho position may have a
783 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
marked accelerating e ect on the rate of deboronation. A 2-formyl group on an arylboronic acid is known to increase the rate of hydrolytic deboronation [80]. Indeed, the coupling of 2- formylphenylboronic acid with 2-iodotoluene at 80 C using Na2CO3 in DME/H2O gives only a 54 % yield of the corresponding biaryl, accompanied by benzaldehyde (39 %). Aprotic conditions are desirable for such boronic acids that are sensitive to aqueous base. Thus, the trimethylene glycol ester of 2-formylphenylboronic acid readily couples with iodotoluene at 100 C in DMF to give the product in 89 % yield, accompanied by less than 10 % of benzaldehyde (Eq. (50)) [78].
ð50Þ
ð51Þ
ð52Þ
Such modified procedures have been utilized by many chemists [81, 82].
Leadbeater and Gri ths developed palladium catalysts for the Suzuki cross-coupling of aryl halides bearing two ortho substituents with phenylboronic acid, and used them in the synthesis of sterically hindered biaryls [83].
Zhang and Chan observed that base has a remarkable accelerating e ect on the rate of Suzuki couplings of sterically bulky boronic acids with halopyridines in non-aqueous solvents (Eq. (53)) [84]. For instance, in the reactions of the extremely sterically bulky arylboronic acid 17 with halopyridines 18a–c, the strong base KOtBu gave the best result among the bases examined (Table 3). During their initial synthetic e orts aimed at obtaining compound 19b, they did not observe any coupling products under the typical Suzuki reaction conditions using either Na2CO3 or NaOEt. Over 70 % of 17 was recovered from the attempted synthesis of 19b using Na2CO3, while a complex reaction mixture was formed using NaOEt. On increasing the strength of the base, boronic acid 17 underwent a Suzuki cross-coupling with 2- bromo-3-methylpyridine (18b), e.g. in the presence of 2.0 equiv. of KOtBu and 5 mol % of Pd(PPh3)4 in DME, to produce 19b in 83 % yield (Table 3).

3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 79
Tab. 3. Base e ect on the cross-coupling of arylboronic acid with halopyridines, yield (%)/reaction time (h).
Base |
19a |
19b |
19c |
|
|
|
|
Na2CO3 |
26/90 |
0/90 |
0/90 |
NaOH |
40/140 |
22/24 |
44/26 |
NaOEt |
74/4 |
0/12 |
45/26 |
KOtBu |
86/4 |
83/16 |
77/10 |
ð53Þ
Generally speaking, stronger bases may give such better yields of coupling products, but the relationship between the base strength and product yield or reaction time should be considered more prudentially.
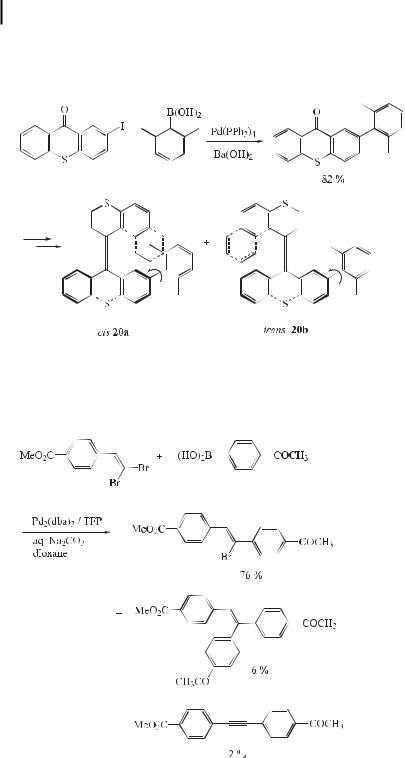
Restricted rotation about the biaryl axis as a result of bulky substituents leads to the existence of atropisomers. Depending upon the degree of steric hindrance due to the ortho substituents, three or four substituents are needed to produce a su cient barrier to rotation at room temperature. This particular form of axial chirality is not generally resistant to heat. To produce acceptable yields of hindered biaryls under Suzuki conditions, high temperatures (60–110 C) [78, 85] and reaction times of several hours are required. In atropisomerselective reactions, these conditions would be deleterious to the discrimination between diastereomeric transition states and could racemize the biaryls formed. As a consequence, it is necessary to carry out such Suzuki reactions at ambient temperature. Recently, conditions employing Pd(OAc)2 and 95 % ethanol were used to generate mono-ortho-substituted biaryls at 20 C (Eq. (54)) [86].
ð54Þ
803 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
In an approach towards a photochemically bistable molecular rotor, the synthesis of the sterically overcrowded isomeric alkenes cis-20a and trans-20b, functionalized with an o-xylyl group as a rotor, has been described (Eq. (55)) [87]. The key step in the synthesis was a Suzuki coupling to attach the xylyl moiety.
ð55Þ
3.2.4
Modified Catalysts and Ligands
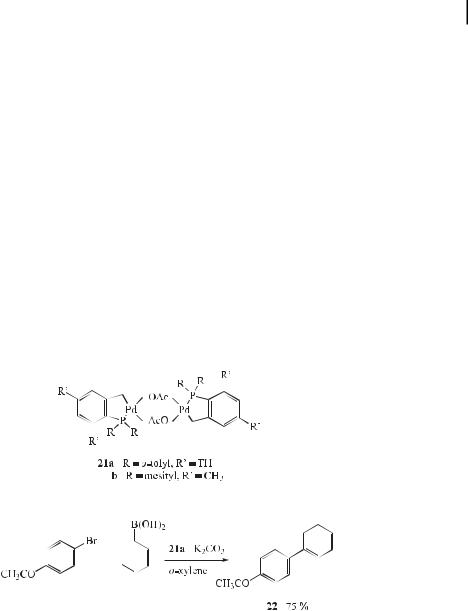
The (E )-bromides in 1,1-dibromo-1-alkenes can be stereoselectively coupled with arylor alkenylboronic acids to give the corresponding (Z )-1-aryl(or alkenyl)-1-bromo-1-alkenes. Tris(2-
ð56Þ
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 81
furyl)phosphine (TFP) is used as a ligand for the palladium, and the combination of 1,4- dioxane and aqueous sodium carbonate produces the best results (Eq. (56)) [88]. Depending on the reaction conditions and reactants, some by-products are obtained. This work demonstrates that the soft ligand TFP is e ective in the palladium-catalyzed coupling of 1,1- dibromo-1-alkenes with organoboronic acids. These modified Suzuki reaction conditions are advantageous compared to the corresponding Stille reaction [89] and the previously reported Suzuki reaction [90].
In recent years, a large number of palladium-mediated Suzuki syntheses of complex synthetic building blocks, as well as of structurally simple but industrially important intermediate products, have been reported and further developed. However, the quality of the catalysts used is generally not su cient for industrial demands. As a result of the increasing importance of unsymmetrically substituted biaryl derivatives, for example, as drug intermediates, the transferability of the catalytic properties of the palladacycle complexes (21a,b) to the cross-coupling between aryl halides and arylboronic acids was examined [91]. It emerged that palladacycles 21 catalyze this type of reaction with unusual e ciency. When 4- bromoacetophenone is treated with phenylboronic acid under the appropriate conditions [bromoacetophenone (10 mmol), phenylboronic acid (15 mmol), K2CO3 (20 mmol), catalyst 21a (0.001 mol %), o-xylene (30 mL), reaction temperature 130 C], the expected coupling product (22) is obtained in 75 % yield, and the turnover number (TON) 75000 is achieved using only 0.001 mol % of 21a as the catalyst (Eq. (57)).
ð57Þ
Najera et al. [92] have reported that for the coupling of aryl halides with organoboronic acids, complexes 23–26 are adequate catalysts, giving TONs between 102 and 106. These palladacycles exhibit greater aerial and thermal stability than palladium(0) complexes.
Most recently, Monteiro et al. have reported that cyclopalladated compounds derived from the ortho-metalation of benzylic tert-butyl thioethers are excellent catalyst precursors for the Suzuki cross-coupling reaction of aryl bromides and chlorides with phenylboronic acid under mild reaction conditions. A broad range of substrates and functional groups are tolerated in this protocol, and high catalytic activity is attained (Eq. (58)) [93].
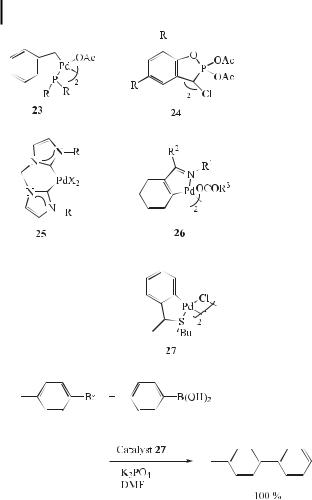
82 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð58Þ
The Suzuki reaction of aryl bromides and chlorides is e ciently catalyzed by palladium/ phosphite complexes generated in situ. The influence of the ligand, base, and various additives was examined. The process tolerates various functional groups, and catalyst turnovers of up to 820,000 were obtained, even with deactivated aryl bromides [94].
The two basic problems of homogeneous catalysis, separation and recycling of the catalyst, can be solved by using two-phase catalysis. Here, the catalyst is in a hydrophilic phase, in which the organic products are insoluble. In order to implement this principle, it is necessary to develop new ligands that are soluble in hydrophilic phases. Diphenylphosphinoacetic acid and the TPPTS ligand (TPPTS, trisodium salt of triphenylphosphane trisulfonate) are used on the ton-scale for the most important industrial two-phase processes. To attain su - cient solubility of the ligands in polar media (particularly water), inorganic groups (sulfonic acid and carboxylic acids, quaternary aminoalkyl/aryl groups, and phosphonium salts) are usually used as substituents on the phosphanes. Beller et al. reported a new class of polar, hydrophilic triarylphosphanes for two-phase catalysis, consisting of aryl-b-O-glycosides of
