
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 83
glucose, galactose, and glucosamine [95]. Thus, the ligand 28 shows a high catalytic activity in the Suzuki reaction (Eq. (59)).
ð59Þ
Palladium-catalyzed Suzuki cross-coupling reactions can be conducted in the ambient temperature ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate (29), in which unprecedented reactivities are witnessed, and which allows easy product isolation and catalyst recycling (Eq. (60)) [96].
ð60Þ
Zhang and Allen [97] have recently reported an easily prepared, airand moisture-stable resin-bound palladium catalyst for the Suzuki coupling reaction. Commercially available resin-bound thiourea, Deloxan THP II [98], was used as the starting material. The resinbound catalyst was prepared simply by treating the wet Deloxan resin with a solution of Pd(OAc)2 in methanol. Suzuki coupling reactions were then carried out using the resin at a

843 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
level equivalent to 2–3 mol % Pd in a mixture of water and isopropanol (Eq. (61)). The reactions proceeded at a similar rate and gave similar yields to those carried out using conventional homogeneous catalysts such as Pd(PPh3)4.
ð61Þ
One limitation to the scope of the Suzuki reaction has been its ine ciency when aryl chlorides are employed as substrates. Recently, Buchwald and Fu have discovered the palladiumcatalyzed cross-coupling of aryl chlorides with organoboron reagents, employing highly active palladium catalysts mediated by special ligands. These are discussed in Section 3.4.
3.2.5
Solid-Phase Synthesis (Combinatorial Methodology)
A traceless solid-phase synthetic strategy has been developed. For example, a solid-phase Suzuki coupling of the Reissert intermediate 30 to 31 has been reported. The process consists of three steps: (a) Solid-phase Reissert formation by the reaction of polymer-supported benzoic acid chloride resin with an isoquinoline, followed by reaction with TMSCN to a ord the aryl bromide of Reissert 30, (b) Suzuki coupling of the solid-phase Reissert 30 with phenylboronic acid to provide the coupling product, and (c) subsequent treatment of the coupling product with aqueous KOH to produce 31 (86 % overall yield based on the starting bromide) (Eq. (62)) [99].
A 9-phenylfluoren-9-yl polystyrene-based resin has been described for the attachment of nitrogen and oxygen nucleophiles. Greater acid stability compared to the standard trityl resins that are widely used in solid-phase peptide synthesis make this solid support an interesting alternative in solid-phase organic synthesis. This resin can be used in Suzuki coupling reactions to furnish biaryls in good yields [100].
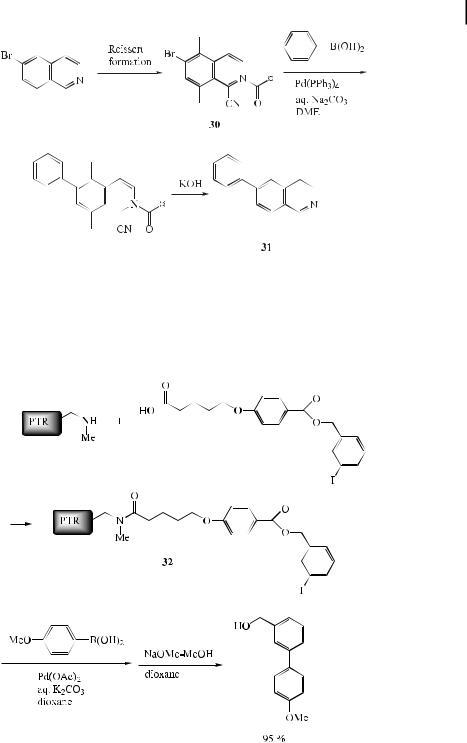
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 85
ð62Þ
The scope and limitations of Pd(0)-mediated coupling reactions between aromatic halides linked to a polystyrene resin and boronic acid derivatives (Suzuki coupling) or arylstannanes (Stille coupling) have been reported. For all the reactions, the conditions were optimized and evaluated with various reagents. In most cases, products were obtained in excellent yields upon cleavage from the solid support (Eq. (63)) [101].
ð63Þ

863 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
It is worthwhile pointing out that coupling results with electron-rich aryltin derivatives (Eq. (65)) were far inferior to those obtained with the corresponding boronic acids (Eq. (64)) [102].
ð64Þ
ð65Þ
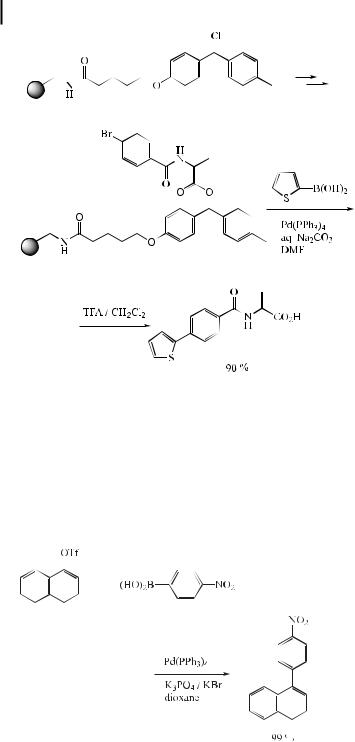
Constructing libraries of nonpolymeric, small organic molecules by solid-phase techniques has been the recent focus of combinatorial synthesis. While many pharmacologically interesting molecules have been prepared on solid supports in the last few years, most of the linkers (e.g., OH, COOH, NHR) employed in these syntheses were inherited from those used in generating peptide, oligonucleotide, and oligosaccharide libraries. E orts to prepare boronic acid derivatives on solid-phase using classical methodology have met with little success. Piettre and Baltzer [103] found that application of Miyaura’s conditions [pinacol ester of diboron (34) (2 equiv.), PdCl2(dppf ) (0.03 equiv.), KOAc (3 equiv.) in DMF at 80 C for 20 h] to a model polymer-bound p-iodobenzamide (33) leads to a solid-phase boronate (35). Subsequent reaction of 35 with an aryl halide such as 36 in the presence of Pd(PPh3)4 (0.02 equiv.)/K3PO4 (5 equiv.)/DMF at 80 C gives 37 in 95 % yield (Eq. (66)) [104].
ð66Þ

3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 87
Tab. 4. Suzuki coupling on solid-phase assisted by microwave irradiation.
In combinatorial chemistry, the reaction times and reaction temperatures required are frequently crucial factors. Microwave irradiation is used to enhance reaction rates [105]. Larhed et al. recently reported that microwave-assisted, palladium-catalyzed coupling of aryl and heteroaryl boronic acids with iodoand bromo-substituted benzoic acids, anchored to TentaGel S RAM, provides coupling products in excellent yields after a reaction time of just 3.8 minutes (45 W) [106]. The preparative results are summarized in Table 4.
Numerous linkers have been developed with the aim of immobilizing substrates on a solid support. Commercially available (G)-a-lipoic acid has been employed as a novel, chemically stable linker for the immobilization of ketones. The utility of this thioacetal-based linker in solid-phase synthesis has been demonstrated by the synthesis of several 4-acetylbiphenyls by means of the Suzuki reaction. The products were readily cleaved from the solid support by treatment with [bis(trifluoroacetoxy)iodo]benzene [PhI(OCOCF3)2] [107].
Recently, the Suzuki reaction has been extensively developed for solid-phase organic chemistry as a method allowing the parallel synthesis of potential medicinal compounds [108]. Chan et al. reported a versatile polymer-supported 4-[4-methylphenyl(chloro)methyl]- phenoxy linker for the solid-phase synthesis of pseudopeptides based on the Suzuki coupling (Eq. (67)) [109].
Franzen has recently presented a review on the Suzuki, Heck, and Stille reactions on solid supports [110].
88 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð67Þ
3.3
Reactions with 1-Alkenyl Halides and Triflates
Arylboronic acids are also e cient reagents for the arylation of 1-alkenyl halides and triflates. The first such cross-coupling reactions between arylboronic acids or esters and 1- alkenyl electrophiles were performed in the presence of a palladium catalyst and a base [111].
ð68Þ

3.3 Reactions with 1-Alkenyl Halides and Triflates 89
It was further discovered that 3-bromochromones readily react with arylboronic acids or their esters under the Suzuki coupling conditions to provide the corresponding isoflavone derivatives in excellent yields (Eq. (69)) [112].
ð69Þ
Stereodefined enol acetates of ketones can readily be synthesized by palladium-catalyzed cross-coupling reactions of arylboron compounds with enol acetates of a-bromo ketones (Eq. (70)) [113].
ð70Þ
Arylcycloalkenes are prepared by cross-coupling with the corresponding triflates [114]. In the arylation of triflates, higher yields can be obtained in the presence of LiCl or LiBr (Eq. (71)).
ð71Þ
The introduction of substituents at the 3-position of pyrrole has been actively pursued in recent years due to the importance of this class of compound in natural product synthesis. In contrast to the significant number of preparations of polysubstituted 3-arylpyrroles, there have been relatively few syntheses of simple 3-arylpyrroles. A literature survey on the preparation of 3-arylpyrroles showed that the methods reported include the reductive cyclization of 2-arylsuccinonitriles, the base-induced ring-closure of arylvinamidinium salts, and

903 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
the rhodium-catalyzed hydroformylation of b-alkynylamines with CO/H2. Recently, a facile synthesis of such 3-arylpyrroles by a tandem Suzuki coupling–dehydrogenation sequence has been reported by Lee and Chung [115]. Thus, the reaction of 1-benzyl-2,5-dihydro-1H- pyrrol-3-yl triflate with arylboronic acids in the presence of a palladium catalyst and base leads directly to the formation of 3-arylpyrroles. Apparently, after Suzuki coupling, subsequent dehydrogenation occurs to provide 3-arylpyrroles. Dehydrogenative aromatization can generally be achieved by heating (300–350 C) hydroaromatic compounds with a catalytic amount of palladium on activated carbon. In the Lee reaction, performed under the Suzuki coupling conditions, the dehydrogenative aromatization of the pyrroline occurs in tandem mode (Eq. (72)).
ð72Þ
A simple preparation of cyclic vinyl boronates has been achieved from vinyl triflates of N- protected tetrahydropyridines. Suzuki coupling of the boronates with aryl bromides, iodides, and triflates proceeds in good yield to give 4-aryl tetrahydropyridines (Eq. (73)) [116].
ð73Þ
Palladium-catalyzed coupling of the 4-chloro- or 4-bromocoumarins (39) with arylboronic acids constitutes an e cient access to 4-arylcoumarins in good yields [117].
ð74Þ

3.3 Reactions with 1-Alkenyl Halides and Triflates 91
Some 3-arylinden-1-ones can be prepared by the Suzuki reaction of pinacol boronate esters with 3-bromoinden-1-ones. Initially, the Suzuki coupling proved to be di cult due to the base sensitivity of the bromides at elevated temperature. Eventually, it was found that the cross-coupling reaction proceeds well when using aqueous NaHCO3 and Pd2(dba)3/PPh3 in DME at 50 C to give the coupling products in relatively good yields (Eq. (75)) [118].
ð75Þ
In the course of a study of structure-based design and synthesis of a potent matrix metallo- proteinase-13 inhibitor based on a pyrrolidinone sca old, the Suzuki coupling reaction of a,b-unsaturated g-lactam iodide (40) with 4-methoxyphenylboronic acid was carried out to give the corresponding coupling product in 77 % yield (Eq. (76)) [119].
ð76Þ
The ubiquitous use of antibiotics for the treatment of infectious disease has resulted in many bacterial strains acquiring resistance to multiple drugs. One of the most serious resistant strains is the methicillin-resistant Staphylococcus aureus (MRSA), which has become a worldwide problem, especially in areas where potent antibiotics have been heavily used. Recently, Merck scientists have reported anti-MRSA activity in carbapenems bearing aromatic substituents at the 3-position. The most recent candidate to emerge from this program is L- 742,728. This compound was prepared in large quantities using the Suzuki cross-coupling as the key reaction by Yasuda et al. (Eq. (77)) [120].
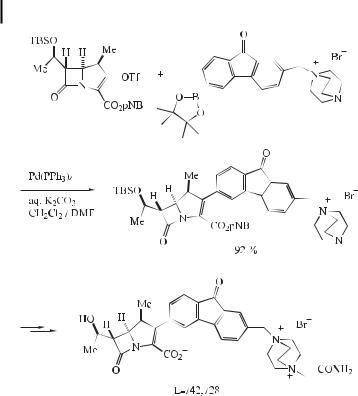
92 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð77Þ
Trifluoromethylated compounds have become increasingly important for a large number of industrial applications, such as pharmaceuticals, agrochemicals, and polymers. Consequently, there is increasing interest in convenient synthetic methods for such compounds. There are two general ways of introducing the trifluoromethyl group: (a) by direct substitution, e.g. by fluorination, halogen-exchange reaction, etc., at a late stage of the synthesis, and
(b) by using trifluoromethyl-substituted building blocks derived from readily available starting materials. As the former route is seldom satisfactory because of low reactivity and low reaction selectivity, the latter is now becoming an important strategy for the preparation of trifluoromethylated molecules because it allow us to obtain these compounds under milder conditions. Deng et al. [121] demonstrated that the Suzuki-type coupling reaction of arylboronic acids with 2-bromo-3,3,3-trifluoropropene in the presence of dichlorobis- (triphenylphosphine)palladium as a catalyst and a base readily gives a-(trifluoromethyl)- styrenes in excellent yields (Eq. (78)).
As mentioned above, palladium-catalyzed couplings of fluoroalkenes are becoming part of the repertoire of organofluorine chemists. However, the range of fluoroalkenes that have been coupled is still very limited and a ubiquitous feature is the presence of a (small) fluorine atom at the a-carbon. Recently, Percy et al. have reported that 1-(N,N- diethylcarbamoyloxy)-2,2-difluoro-1-iodoethene undergoes smooth Suzuki coupling (Eq. (79)) [122].
