
Astruc D. - Modern arene chemistry (2002)(en)
.pdf
53
3
The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
Akira Suzuki
Abstract
Carbon–carbon bond formation reactions are important processes in chemistry, constituting key steps in the building of complex molecules from simple precursors. Recently, such couplings have been realized by cross-coupling reactions between organoboron compounds and organic electrophiles in the presence of a palladium catalyst and a base. This chapter surveys couplings of arylor heteroarylboron compounds with aryl or heteroaryl halides, including chlorides and triflates, which selectively a ord the corresponding biaryl derivatives in excellent yields. The application of these couplings in polymer chemistry is also mentioned.
3.1
Introduction
Carbon–carbon bond-forming reactions are among the most important processes in chemistry, providing key steps in the building of more complex molecules from simple precursors. Over several decades, reactions enabling carbon–carbon bond formation between molecules with saturated sp3 C-atoms have been developed. However, there were no simple and general methods for establishing carbon–carbon linkages between unsaturated species such as vinyl, aryl, and alkynyl moieties prior to the discovery and development of metal-mediated cross-coupling reactions in the 1970s.
In the mid-1970s, attempts were made to find a stereoand regioselective synthetic means of obtaining conjugated alkadienes, which are of great importance in organic chemistry, both in themselves as well as with regard to their utilization in other reactions such as the Diels– Alder reaction. A number of methods for the preparation of conjugated dienes and polyenes were developed utilizing organometallic compounds. Although each of these methods had its own merits, the scope of many of these reactions was limited by the nature of the organometallic involved or the procedure employed. Perhaps the most promising procedure for preparing conjugated dienes or enynes in a selective manner was considered to be that based on the direct cross-coupling reaction of stereodefined haloalkenes or haloalkynes in the presence of a transition metal catalyst with stereodefined alkenylboron compounds, which are readily prepared from alkynes by hydroboration. In spite of e orts by many chemists to find such coupling reactions, there were no successful reports when we started this work.
Modern Arene Chemistry. Edited by Didier Astruc
Copyright 8 2002 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN: 3-527-30489-4

54 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
At the initial stage of our exploration, we surmised that the di culties encountered in achieving such a coupling stemmed from the following features. The common mechanism of transition metal catalyzed cross-coupling reactions between organometallic compounds and organic halides involves a sequence of: (a) oxidative addition, (b) transmetalation, and (c) reductive elimination [1]. One of the major reasons why 1-alkenylboranes cannot react with 1-alkenyl- or 1-alkynyl halides appears to be step (b), where the transmetalation process between RMX (M ¼ transition metal, X ¼ halogen) and organoboranes does not occur readily because of the weak carbanion character of organic groups in the organoboranes. To overcome this di culty, we envisaged the use of tetracoordinated organoboron compounds instead of tricoordinated organoboron derivatives. It was observed that the methyl group in tetramethylborate is 5.5 times more electronegative than the methyl groups of trimethylborane [2]. Such speculation is also expected to apply in the case of the reaction of triorganoboranes in the presence of base. Actually, we found that the cross-coupling reaction of vinylic boron compounds with vinylic halides proceeds smoothly in the presence of base and a catalytic amount of a palladium complex to give the expected conjugated alkadienes and alkenynes stereoand regioselectively in excellent yields [3]. Not only such vinylborane derivatives but also arylboron compounds readily cross-couple with a number of organic electrophiles to selectively provide coupling products in high yields. In this chapter, such crosscoupling reactions of arylboron compounds are described.
3.2
Reactions with Aryl Halides and Triflates: Synthesis of Biaryls
3.2.1
Aromatic–Aromatic Coupling
As mentioned in the previous section, it was found that vinylboron compounds (sp2 carbon– boron bond) readily react with vinylic halides (sp2 carbon–halogen bond) to stereoand regioselectively provide the corresponding cross-coupling products, i.e. conjugated alkadienes, in excellent yields. In order to confirm the possibility of cross-coupling between areneboronic acids and aryl halides, both of which possess sp2 carbons, such coupling reactions were subsequently investigated.
The first observation concerning the preparation of biaryls was reported in 1981 [4]. Thus, the palladium-catalyzed cross-coupling reaction of phenylboronic acid with a number of haloarenes was found to proceed smoothly in the presence of base to selectively a ord the corresponding biaryls in high yields (Eq. (1) and Table 1).
ð1Þ
Following this discovery, various modifications were made to the reaction conditions. A combination of Pd(PPh3)4 or PdCl2(PPh3)2 and aqueous Na2CO3 in dimethoxyethane (DME) was reported to work satisfactorily in most cases [5, 6].
|
|
|
3.2 |
Reactions with Aryl Halides and Triflates: Synthesis of Biaryls |
55 |
||
Tab. 1. Synthesis of Biphenylsa. |
|
|
|
|
|||
|
|
|
|
||||
|
|
|
Solvent |
Reac. time (h) |
Yield of biphenyl (%) |
||
|
|
|
|||||
|
|
|
|
|
|
|
|
o-MeC6H4Br |
benzene |
6 |
94 |
|
|
||
p-MeC6H4Br |
benzene |
6 |
88 |
|
|
||
o-MeOC6H4Br |
benzene |
6 |
99 |
|
|
||
p-ClC6H4Br |
benzene |
6 |
89 |
|
|
||
p-MeO2CC6H4Br |
benzene |
6 |
94b |
||||
Mesityl bromide |
toluene |
17 |
80b |
|
|||
a The reaction was carried out by using 10 % excess of phenylboronic acid, Pd(PPh3)4 (3 mol %), and 2 M Na2CO3-H2O (2 equiv.) at the reflux temperature of solvent.
b Isolated yields.
Combinations with other bases, such as Et3N [7, 8], NaHCO3 [9], Cs2CO3 [10], Tl2CO3 [11], and K3PO4 [12], with or without Bu4NCl [13] and 18-crown-6 [10], were also used. The reaction is successful for aryl iodides, bromides, and triflates. Chlorobenzene derivatives are generally quite inert to oxidative addition, but some p-deficient heteroaryl chlorides and related compounds do give coupling products [14]. Most recently, modified conditions e ective for aryl chlorides have been proposed (see Section 3.4). The reaction proceeds most rapidly under homogeneous conditions (aqueous base in DME), but reasonable yields are also obtained under heterogeneous conditions. For example, K2CO3 suspended in toluene works well for base-sensitive reactants [15]. Although the conditions using such bases are not entirely compatible with the functional groups present in all desired reactants, the extremely mild conditions using CsF or Bu4NF allow the synthesis of various functionalized biaryls (Eq. (2)) [16].
ð2Þ
Phosphine-based palladium catalysts are generally used since they are stable to prolonged heating; however, extremely high coupling reaction rates can sometimes be achieved by using palladium catalysts without a phosphine ligand, such as Pd(OAc)2, [(h3-C3H5)PdCl]2, or Pd2(dba)3C6H6 (Eq. (3)) [17, 18].
ð3Þ
563 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
Cross-coupling reactions of areneboronic acids with aryl triflates in the presence of K3PO4 and a catalytic amount of Pd(PPh3)4 or PdCl2(dppf ) in dioxane proceed with high yields. The reaction conditions are su ciently mild that a variety of functionalized biaryls may readily be obtained (Eq. (4)) [19].
ð4Þ
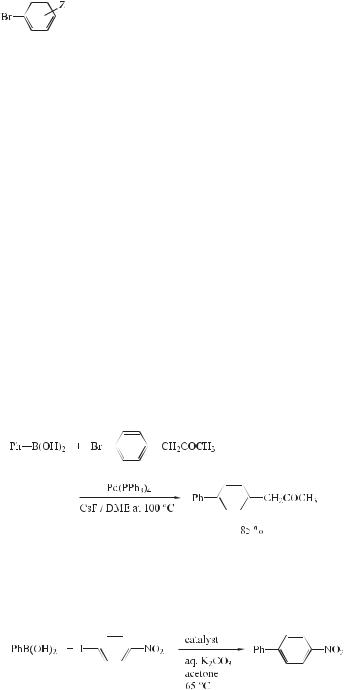
Aromatic–aromatic coupling reactions have been amply applied in syntheses of biologically active chemicals, and of other materials of interest in many scientific and industrial fields. Terprenin was recently discovered in the fermentation broth of Aspergillus candidus. The impressive feature of the immunosuppressive activities of terprenin is its highly potent in vitro and in vivo suppressive e ect on immunoglobulin E antibody production without any toxicological signs. The total synthesis of terprenin was reported recently, the key steps of which relied on the Suzuki reaction (Eq. (5)) [20]. The highly e cient and practical production of this important natural product o ers promise for the development of a new type of antiallergic drug.
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 57
(5)
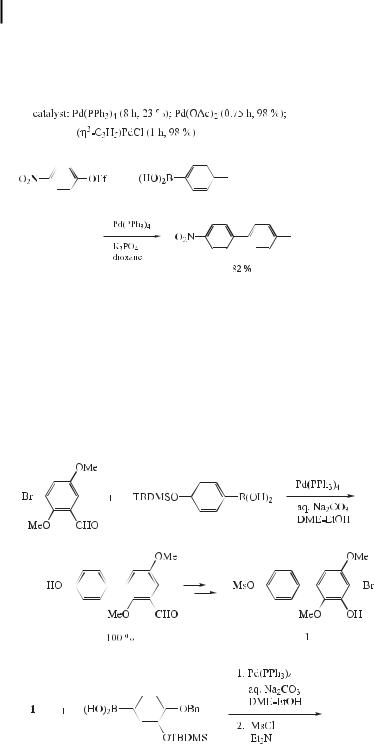
Robustaflavone, a naturally occurring compound, is an inhibitor of hepatitis B virus replication in vitro. The natural material was isolated from the seed kernels of Rhus succedanea. To provide ready access to su cient quantities of material for continued biological studies, as well as to provide a general route for the preparation of structural analogues, a total synthesis of robustaflavone was requested, and Zembower recently reported the first total synthesis of this compound using Suzuki coupling as a key step (Eq. (6)) [21].
ð6Þ
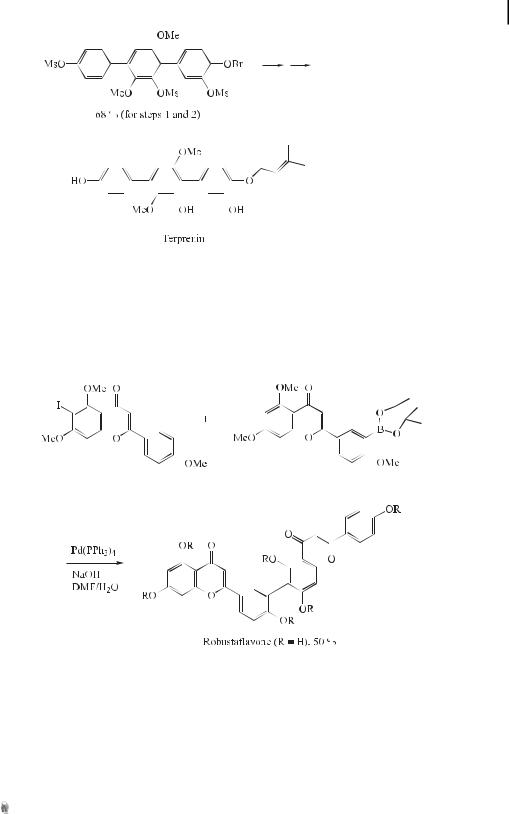
A number of dimeric carbazole alkaloids have been isolated from various natural sources in recent years, which have been found to exhibit various biological activities including antitumor, anti-inflammatory, and cytotoxic activities. In 1996, clausenamine A was isolated from the stem and root bark of Clausena excavata, which is used in Chinese herbal medicine for detoxification treatment following poisonous snakebites. The first total synthesis of clau-
583 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
senamine A has since been developed, which involves Suzuki cross-coupling and an oxidative coupling reaction (Eq. (7)) [22].
ð7Þ
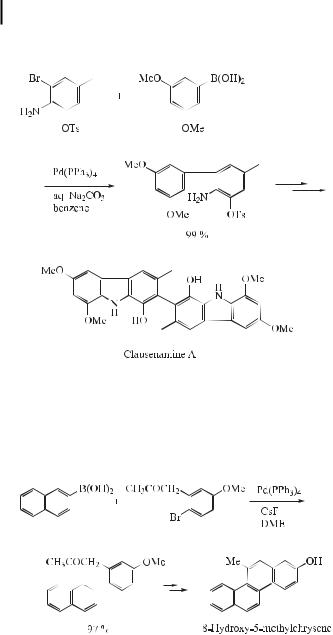
The Suzuki cross-coupling reaction is recognized as a novel, abbreviated method for the synthesis of 2-hydroxychrysene, 2-hydroxy-5-methylchrysene, and 8-hydroxy-5-methyl- chrysene from easily accessible reactants (Eq. (8)) [23]. These phenolic compounds constitute precursors for the synthesis of dihydrodiol and bay-region diol epoxide derivatives of chrysene and 5-methylchrysene, which are implicated as the active forms of carcinogenic polynuclear aromatic hydrocarbons.
ð8Þ
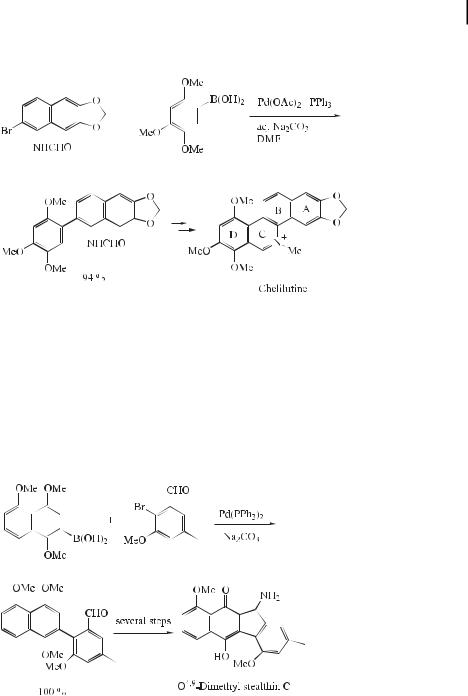
The benzo[c]phenanthridines are an important group of isoquinoline alkaloids, many of which show a wide range of pharmacological properties including antitumor and antiviral activities. They have therefore attracted much synthetic interest in the 60 years since Robinson published the first synthesis. Geen et al. reported a synthesis involving a late-stage fusion of the A and D ring components, thereby allowing easy access to a number of benzo[c]phenanthridine alkaloids from only a few synthetic intermediates [24]. For example, following the coupling of 2-bromo-1-formamidonaphthalene with 2,4,5-trimethoxyphenyl-
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 59
boronic acid to a ord the desired coupling product in 94 % yield, chelilutine can readily be obtained after a few more steps (Eq. (9)).
ð9Þ
Comins and Green have recently developed a new method for the enantioselective synthesis of 4-substituted phenylalanines based on Pd-catalyzed cross-coupling reactions of a protected boronophenyl alanine with aromatic halides. These reactions proceed with good to excellent yields, and furnish the desired products with high enantiomeric purities [25].
Recently, a growing number of radical scavengers have been isolated from microorganisms. In 1992, for example, Seto et al. reported the isolation of stealthins A and B as potent radical scavengers from Streptomyces viridochromogenes, and showed that their radicalscavenging activities were 20–30 times greater than that of vitamin E. Most recently, O4;9- dimethylstealthin C [26a] and O12-acetyl-O4,O9-dimethylstealthin A [26b] have been synthesized using the Suzuki coupling as a key step, as shown in Eq. (10).
ð10Þ
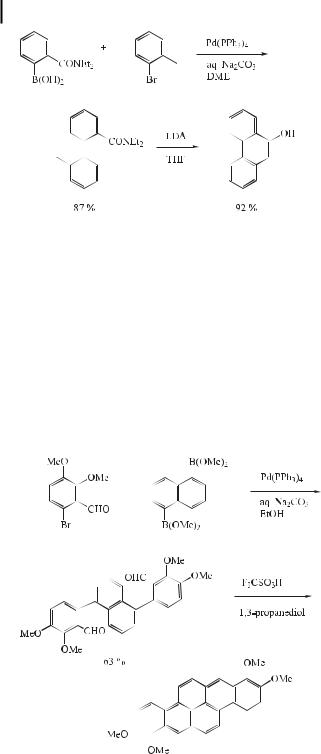
Snieckus and Fu reported a new general and regiospecific synthesis of 9-phenanthrols involving direct ortho-metalation, Suzuki cross-coupling, and an LDA-mediated directed remote metalation sequence (Eq. (11)) [27].
60 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð11Þ
A versatile method for the synthesis of complex, fused polycyclic aromatic systems in high chemical yield has been discovered. Pd-catalyzed Suzuki-type cross-coupling allows the preparation of nonfused skeletal ring systems in high yield. The ring-forming step, which generally proceeds in high yield, utilizes 4-alkoxyphenylethynyl groups and is induced by strong electrophiles such as trifluoroacetic acid and iodonium tetrafluoroborate. The reaction produces phenanthrene moieties, which are integrated into extended polycyclic aromatic structures [28, 29]. Fused polycyclic benzenoids as well as benzenoid/thiophene systems may be prepared utilizing this methodology.
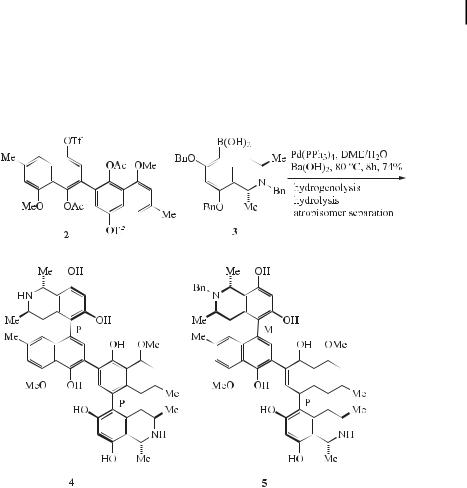
A new synthetic approach to polycyclic aromatic compounds has been developed based on double Suzuki coupling of polycyclic aromatic hydrocarbon bis(boronic acid) derivatives with o-bromoaryl aldehydes to furnish aryl dialdehydes. These are then converted to larger polycyclic aromatic ring systems by either: (a) conversion to diolefins by Wittig reaction followed by photocyclization, or (b) reductive cyclization with trifluoromethanesulfonic acid and 1,3- propanediol (Eq. (12)) [30].
ð12Þ
3.2 Reactions with Aryl Halides and Triflates: Synthesis of Biaryls 61
Recently, the anti-HIV alkaloids michellamines A (4) and B (5) have attracted much attention. The tetraaryl skeleton of the michellamines is constructed by first forming the inner (nonstereogenic) biaryl axis and then adding the two other (stereogenic) axes by means of a double Suzuki-type cross-coupling reaction between binaphthalene ditriflate (2) and isoquinolineboronic acid (3) (Eq. (13)) [31].
ð13Þ
Another total synthesis of michellamines A–C, korupensamines A–D, and ancistrobrevine B has been developed by Hoye et al. [32], who emphasized that Suzuki coupling of the boronic acid derived from the naphthalene moiety with a THIQ-iodide proved to be the most generally e ective method for forming the hindered biaryl bond. A racemic isochromane analogue of michellamines has been synthesized by a similar procedure [33].
Vancomycin is well-known as the last line of defense against drug-resistant bacteria. Nicolaou and his group reported a Suzuki coupling approach to the AB-COD bicyclic system of vancomycin [34]. The total synthesis of the vancomycin aglycon was subsequently reported by the same workers [35].
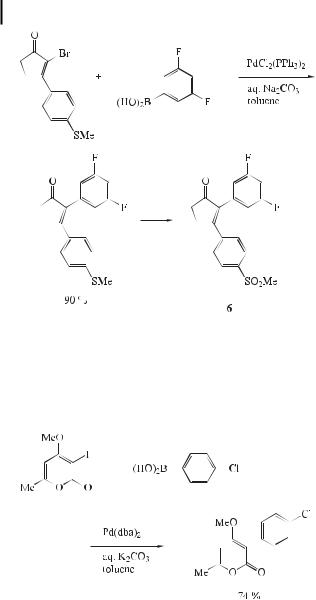
It has been demonstrated that 2-(30,50-difluorophenyl)-3-(40-methylsulfonylphenyl)- cyclopent-2-enone (6), which is synthesized by means of a Suzuki reaction (Eq. (14)), displays high selectivity and potency towards cyclooxygenase [36].
The 4-hydroxy-2H-pyran-2-one moiety is a key structural feature in a recently discovered new class of non-peptidic HIV protease inhibitors. Therefore, the synthetic methodologies were extended to allow the functionalization of commercially available chemicals. Direct arylation of the pyrone ring at the C-3 and C-5 positions had not been reported until the re-
62 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð14Þ
cent publication of the palladium-catalyzed cross-coupling of organozinc derivatives with iodobenzene [37]. Recently, Pleixats et al. demonstrated that 3-arylpyrones and 5-arylpyrones, incorporating the 4-methoxy-2H-pyran-2-one moiety, are readily obtained by Suzuki crosscoupling of the corresponding 3-iodo and 5-iodo derivatives with arylboronic acids (Eq. 15) [38].
ð15Þ
Diospyrin was first isolated in 1961 as an orange-red constituent of Diospyros montana Roxb. (Ebenaceae) in India. Mori and Yoshida have reported the first synthesis of diospyrin, employing Suzuki coupling as a key reaction enabling connection of the two 7-methyljuglone units (Eq. (16)) [39].
3,7-Dihydroxytropolone derivatives have emerged as the foremost representatives of a new class of potent, competitive inhibitors of inositol monophosphatase. The first successful preparations of monoand disubstituted 3,7-dihydroxytropolones were accomplished by single or double Suzuki coupling reactions between these permethylated monobromoand
