
Astruc D. - Modern arene chemistry (2002)(en)
.pdf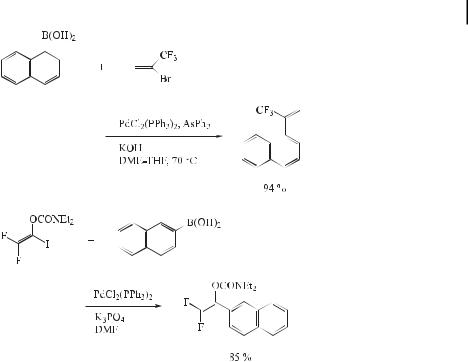
3.4 Reactions with Aryl Chlorides and Other Organic Electrophiles 93
ð78Þ
ð79Þ
3.4
Reactions with Aryl Chlorides and Other Organic Electrophiles
One of the challenges in the Suzuki-type cross-coupling is to extend this reaction from electron-rich aryl iodides, bromides, and triflates to less reactive aryl sulfonates and aryl chlorides, which show poor reactivity in terms of oxidative addition in the catalytic cycle. Aryl mesylates, benzenesulfonates, and tosylates are much less expensive than triflates, and are unreactive toward palladium catalysts. The Ni(0)-catalyzed Suzuki-type cross-coupling reaction of aryl sulfonates, including mesylates, with arylboronic acids in the presence of K3PO4 has been reported [123].
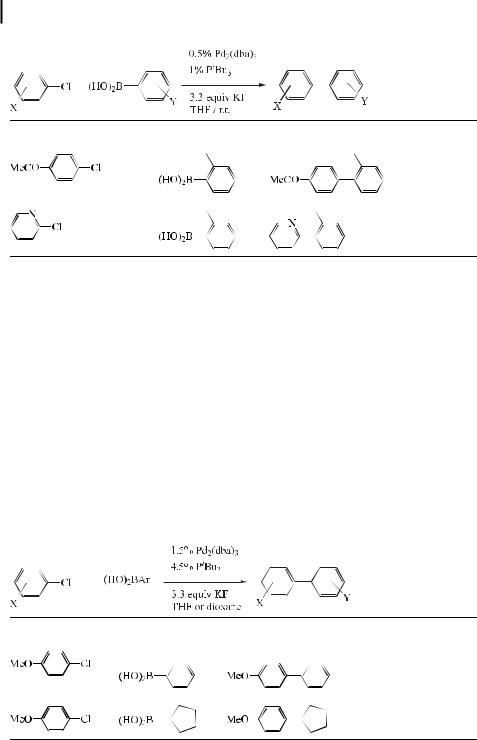
One limitation in the scope of the Suzuki reaction has been its ine ciency when aryl chlorides are employed as substrates, although there have been several accounts of the coupling of electron-poor aryl chlorides [124].
The low reactivity of aryl chlorides in cross-coupling reactions is generally ascribed to their reluctance to oxidatively add to Pd(0). Aryl halides bearing an electron-withdrawing group oxidatively add to Pd(0) more readily than do the corresponding unactivated aryl halides [125]. In view of the greater availability and lower cost of aryl chlorides relative to aryl bromides, iodides, and triflates, many chemists have shown an interest in new methodologies employing aryl chlorides.
Most recently, Fu and co-workers [126] have reported that the use of Pd2(dba)3/PtBu3 as a catalyst for a wide range of aryl and vinyl halides, including chlorides, facilitates their Suzuki cross-coupling with arylboronic acids, giving the coupled products in very good yield, typi-
943 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
Tab. 5. Suzuki couplings of activated aryl chlorides.
(Hetero)Aryl Chloride |
Boronic Acid |
Product |
Yield (%) |
|
|
|
|
99
97
cally at room temperature. Using Pd(OAc)2/Pcy3, a diverse array of aryl and vinyl triflates react cleanly at room temperature. These two catalyst systems cover a broad spectrum of commonly encountered substrates for Suzuki couplings. Furthermore, they display novel reactivity patterns, such as the selective cross-coupling by Pd2(dba)3/PtBu3 of an aryl chloride in preference to an aryl triflate, and they can be used at low loadings, even for the reactions of aryl chlorides. Some representative results are presented in Tables 5, 6, and 7.
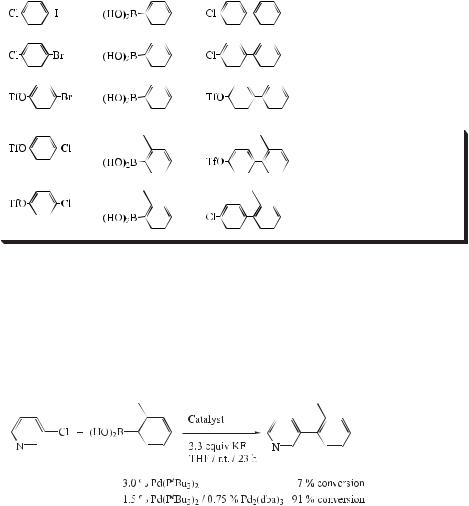
To gain some insight into the Pd2(dba)3/PtBu3 catalyst system, Fu et al. pursued a series of NMR and reactivity studies, and found that the bis(phosphine) adduct, Pd(PtBu3)2, is favored over the monophosphine and the tris(phosphine) over a wide range of PtBu3:Pd ratios. They monitored the Suzuki coupling of 3-chloropyridine and 2-tolylboronic acid by 31P NMR spectrometry (2.5 % Pd2(dba)3, 5 % PtBu3; [D8]THF), and found Pd(PtBu3)2 to be the sole species observed during the course of the reaction. Since the overall PtBu3:Pd ratio is 1:1,
Tab. 6. Suzuki couplings of unactivated aryl chlorides.
Aryl Chloride |
Boronic Acid |
Product |
Temp. ( C) |
Yield (%) |
70 88
100 75
|
3.4 |
Reactions with Aryl Chlorides and Other Organic Electrophiles |
|
95 |
||
Tab. 7. Chemoselective Suzuki couplings. |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
Aryl Halide |
Boronic Acid |
Product |
Conditions |
Yield (%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.5 % Pd2 |
(dba)3 |
98 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.2 % PtBu3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.5 % Pd2 |
(dba)3 |
97 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.2 % PtBu3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.5 % Pd2 |
(dba)3 |
98 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.2 % PtBu3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
this suggests that one-half of the palladium is in the form of Pd(PtBu3)2, while the other half is in the form of a phosphine-free complex.
Pd(PtBu3)2 does not appear to be the active catalyst in the Suzuki coupling process under Fu’s conditions, since the reaction of 3-chloropyridine with 2-tolylboronic acid proceeds sluggishly at room temperature. However, the addition of phosphine-free Pd2(dba)3 to the Pd(PtBu3)2 leads to a marked increase in the rate of cross-coupling (Eq. (80)).
ð80Þ
These observations suggest that a palladium monophosphine adduct may be the active catalyst in these Suzuki couplings, and that phosphine-free palladium complexes present in the reaction mixture may play an important role by increasing the concentration of the active catalyst. The unusual cross-coupling activity promoted by PtBu3 may therefore be attributed to both its size and its electron-richness: the steric demand favors dissociation (relative to less bulky phosphines) to a monophosphine complex, which, due to the donating ability of PtBu3, readily undergoes oxidative addition.
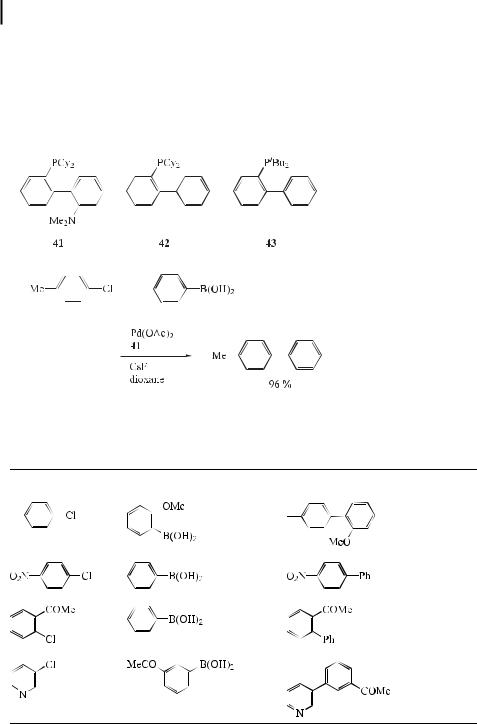
Buchwald [127] and Lohse [128] have independently reported coupling reactions using aryl chlorides. In order to overcome the sluggish oxidative addition step of aryl chlorides in
963 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
their palladium-catalyzed CaN bond-forming reaction, Buchwald et al. have explored the use of electron-rich phosphine ligands. They were finally able to demonstrate that use of the aminophosphine ligand 2-(dimethylamino)-20-dicyclohexylphosphino-1,10-biphenyl (41) is superior and significantly expands the scope of the palladium-catalyzed amination of unactivated aryl chlorides. They also observed that the reaction conditions using Pd(OAc)2/41/ CsF are significantly more e ective for the Suzuki coupling of unactivated aryl chlorides with arylboronic acids (Eq. (81)).
ð81Þ
Thereafter, Buchwald et al. [129] reported that mixtures of palladium acetate and o-(di-tert- butylphosphino)biphenyl (43) (see Table 8) catalyze the room temperature Suzuki coupling
Tab. 8. Suzuki coupling of aryl chlorides under Buchwald’s conditionsa.
Chloride |
Bopronic Acid |
Product |
Yield (%) |
|
|
|
|
95
98
93
94
a Reaction conditions: catalyst Pd(OAc)2, ligand 43 (2L/Pd), KF in THF.
3.4 Reactions with Aryl Chlorides and Other Organic Electrophiles 97
of aryl bromides and aryl chlorides at a level of 0.5–1.0 mol % Pd, and that the use of o- (dicyclohexylphosphino)biphenyl (42) allows Suzuki couplings to be carried out at low catalyst loadings (0.000001–0.02 mol % Pd). The process tolerates a broad range of functional groups and substrate combinations, including the use of sterically hindered substrates. The following conclusions were made. The catalyst systems based on ligands 42 and 43, which are easily prepared in one step and are commercially available [130], are new, highly active catalysts for the Suzuki coupling of aryl halides. While the use of 43 generally gives faster reaction rates in room temperature Suzuki couplings, 42 is more e ective for hindered substrates and operates more e ciently at low catalyst loadings. Of great importance is the fact that using such ligands the rate of oxidative addition is greatly enhanced, while the rates of the other steps of the catalytic cycle are probably also increased. Thus, their use avoids the common pitfall whereby speeding up the rate of one step slows that of another, resulting in little or no increase in the overall rate of the reaction. The authors consider that the success of these ligands is due to a combination of (1) their electron-richness, which enhances the rate of oxidative addition and keeps the palladium in solution, and (2) their steric bulk, which enhances the rate of reductive elimination and maximizes the concentration of L1Pd complexes, thereby increasing the rate of transmetalation. It is also being considered that (3) the presence of the o-biphenyl moiety might be important, helping to confer aerial stability to the ligand, enhancing the rate of reductive elimination, as well as stabilizing the catalyst by interacting with the Pd. However, further mechanistic studies to determine the factors responsible for the high activities of these catalysts are necessary.
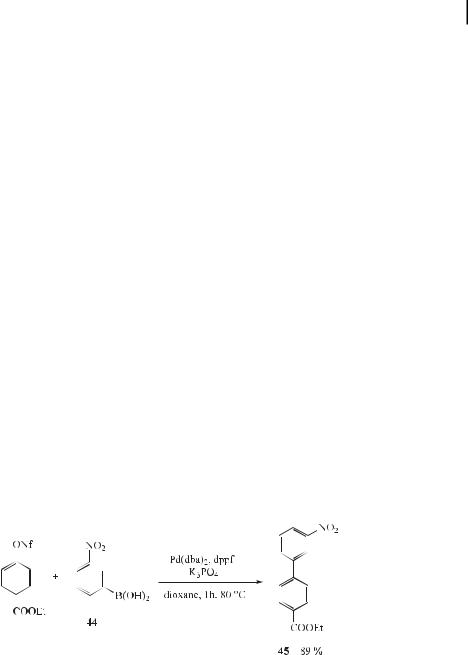
Due to the moderate reactivity of aryl triflates and the high cost of the triflate functionality, aryl fluoroalkanesulfonates [ArOSO2(CF2)nCF3] have recently been proposed as an alternative to triflates, because they are easily prepared using commercially available fluoroalkanesulfonic anhydrides or halides. Especially attractive are aryl nonaflates (ArONf ¼ ArOSO2(CF2)8CF3), which are readily prepared and are stable to flash column chromatography. Rottla¨nder and Knochel [131] reported that treatment of the nonaflate with the boronic acid 44 provides, under typical conditions for Suzuki coupling, the expected product (45) in 89 % yield (Eq. (82)).
ð82Þ
As an alternative to the use of organic electrophiles, Kang et al. employed hypervalent iodonium compounds in cross-couplings with organoboranes [132]. Thereafter, these authors reported on cross-coupling reactions of organoboron compounds with organolead(IV) compounds [133]. Of the catalysts tested, Pd2(dba)3 CHCl3, Pd(OAc)2, and PdCl2,

983 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
Pd2(dba)3 CHCl3 was generally found to be superior. A solvent system of CH3CN/DME (1:1) proved to be most suitable. Also, it is noteworthy that CuI (10 mol %) was added as a cocatalyst to avoid homocoupling.
3.5
Miscellaneous
Kabalka et al. have reported a solvent-free Suzuki coupling methodology involving the use of a commercially available potassium fluoride/g-alumina mixture, doped with a ligand-free Pd(0) catalyst (Eq. (83)) [134]. A number of parameters of the reaction were investigated. Suzuki reactions require the presence of a base, which is also true of the solid-state reaction. Both potassium phosphate and potassium fluoride proved e ective in inducing the solidphase coupling reactions. The quantity of palladium was found to be important; a 4–5 % loading of palladium was most e ective.
ð83Þ
There is currently a great deal of interest in performing chemical reactions under solventfree conditions, since there are both financial and environmental benefits. One problem associated with performing reactions with solid starting materials is the mixing of the reactants. Another is the di culty of achieving uniform and controlled heating of large amounts of powdery materials. Such di culties may be overcome by a ball-milling procedure, and the Suzuki coupling reaction has been attempted under these conditions [135]. A factor that needs to be considered for the ball-mill conditions is the degree of stickiness of the mixture. Peters et al. have observed that the addition of sodium chloride to the reaction mixtures is e cient in making them su ciently powdery. Sodium chloride has the following favorable characteristics: hard and brittle crystals, chemical inertness, good water solubility, low toxicity, and low price. The order of reactivity is complementary to that of the normal Suzuki reaction (Eq. (84)).
ð84Þ

3.6 Applications in Polymer Chemistry 99
3.6
Applications in Polymer Chemistry
Aromatic, rigid-rod polymers play an important role in a number of diverse technologies, including high-performance engineering materials, conducting polymers, and nonlinear optical materials.
Constitutionally homogeneous oligo-p-phenyls are materials of considerable current interest to chemists, physicists, and material scientists because such compounds are excellent model compounds for developing a profound understanding of the spectroscopic and redox properties of polyaromatic systems, and of the thermal phase behavior and solution properties of rod-like liquid-crystalline molecules. Furthermore, functionalized oligo-p-phenyls have gained some importance as mainchain-sti ening building blocks in semi-flexible polymers such as aromatic polyesters and polyimides. Despite considerable advantages, parent oligo-p- phenyls have a serious drawback, however, with regard to the above applications: their solubility decreases dramatically with increasing number of benzene rings. Fortunately, it is known that the attachment of lateral methyl groups to oligo-p-phenyls increases their solubility. Nevertheless, the solubilizing e ect of methyl groups is insu cient in the case of longer oligo-p-phenyls, and flexible side chains are required to further increase the solubility of rigid-rod molecules such as aromatic polyesters. By taking advantage of this latter concept, Galda and Rehahn [136] applied the Suzuki coupling as the oligomer formation reaction, which was found to furnish the expected oligo-p-phenyls in excellent yields (Eq. (85)).
ð85Þ
Many poly(p-phenylenes) have since been synthesized by such Suzuki reactions [137].
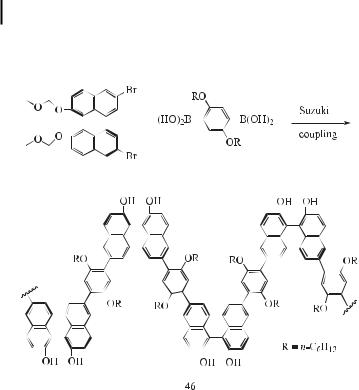
The development of enantioselective polymer catalysts is important with regard to the efficient production of optically pure chiral organic compounds, including drug molecules. The major advantage that a polymer catalyst o ers is the ease of recovery and reuse of the expensive catalyst. The use of polymer catalysts may also enable the reactions to be carried out in flow reactors or flow membrane reactors. Recently, a highly enantioselective hetero- Diels–Alder reaction catalyzed by chiral polybinaphthylaluminum complexes was reported [138]. Thus, Jorgensen et al. synthesized the optically active polybinaphthyl 46 as a polymeric ligand by Suzuki coupling of the optically active binaphthyl dibromide and a benzenediboronic acid derivative (Eq. (86)). The polymer 46 reacts with Me3Al or Me2AlCl to
1003 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
generate the corresponding polymeric chiral aluminum catalyst in situ for the hetero Diels– Alder reaction.
ð86Þ
Research on dendrimers was first focused on synthetic aspects concerning this new class of macromolecules, and a broad range of dendrimers is now available, some even commercially. The emphasis of current study has shifted from merely synthetic aspects to questions of practical applications of dendrimers and the ways in which these unique compounds are superior to known systems. Consequently, much more useful synthetic procedures are required. Schlu¨ter et al. have reported the synthesis of dendrimers with poly(p-phenylene) (PPP) derived cores using the Suzuki polycondensation [139].
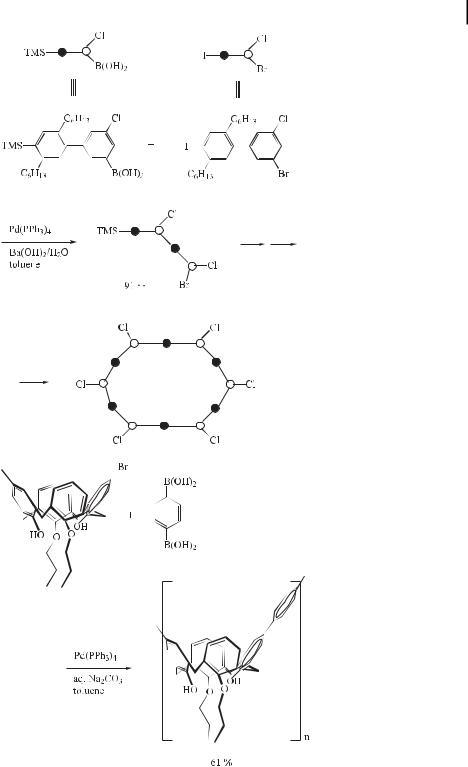
The synthesis of a variety of kinked oligophenylene building blocks has recently been demonstrated. The synthetic strategy is repetitive, utilizes the Suzuki cross-coupling protocol, and involves potentially bifunctional building blocks (modules) that bear one functional group for coupling and another that serves as place holder. After the coupling, the place holder is converted into a coupling functionality for the next growth step. The building blocks also bear chloro substituents, which remain una ected during each transformation. For one of the large building blocks, it has been shown that it may be cyclized to an oligophenylene macrocycle with a chloro substituent at each corner and a pair of hexyl chains at each side (Eq. (87)) [140].
Dondoni et al. have recently explored the synthesis of oligomers in which 1,3-(distal)- calix[4]arenes are linked to a p-phenylene unit by a single carbon–carbon bond emerging from the upper rim of the macrocycle (Eq. (88)) [141].
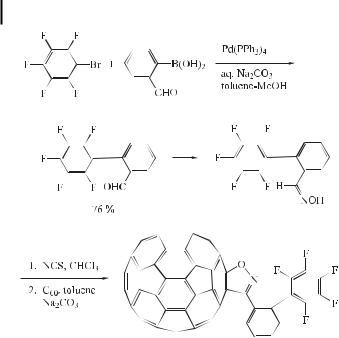
Investigations concerning the synthesis and electrochemical behavior of a new class of acceptor-substituted isoxazolofullerenes have been reported. In this instance, such fullerene derivatives were synthesized by Suzuki coupling (Eq. (89)) [142].
3.6 Applications in Polymer Chemistry 101
ð87Þ
ð88Þ
102 3 The Suzuki Reaction with Arylboron Compounds in Arene Chemistry
ð89Þ
A novel topological strategy has been examined for designing amorphous molecular solids suitable for optoelectronic applications. In this approach, chromophores were attached to a tetrahedral point of convergence. For instance, stilbenoid units were covalently linked to a tetraphenylmethane core by means of a palladium-catalyzed Suzuki coupling reaction [143]. The optical properties of these compounds were examined.
References
1 |
F. Diederich, P. J. Stang (Eds.), Metal- |
4 |
N. Miyaura, T. Yanagi, A. Suzuki, Synth. |
|
Catalyzed Cross-Coupling Reactions, Wiley- |
|
Commun. 1981, 11, 513. |
|
VCH, Weinheim, 1998. |
5 |
S. Gronowitz, V. Bobosik, K. Lawitz, |
2 |
O. Gropen, A. Haaland, Acta Chim. |
|
Chem. Scripta 1984, 23, 120. |
|
Scand. 1973, 27, 521. |
6 |
B. I. Alo, A. Kandil, P. A. Patil, M. J. |
3 |
(a) A. Suzuki, Pure Appl. Chem. 1985, 57, |
|
Sharp, M. A. Siddiqui, V. Snieckus, J. |
|
1749. (b) A. Suzuki, Pure Appl. Chem. |
|
Org. Chem. 1991, 56, 3763. |
|
1994, 66, 213. (c) N. Miyaura, A. Suzuki, |
7 |
W. J. Thompson, J. Gaudino, J. Org. |
|
Chem. Rev. 1995, 95, 2457. (d) A. Suzuki, |
|
Chem. 1984, 49, 5237. |
|
in Metal-Catalyzed Cross-Coupling Reactions |
8 |
W. Mu¨ller, D. A. Lowe, H. Neijt, S. |
|
(Ed.: F. Diederich, P. J. Stang), Wiley- |
|
Urwyler, P. Herrling, D. Blaser, D. |
|
VCH, Weinheim, 1998, p. 49. (e) A. |
|
Seebach, Helv. Chim. Acta 1992, 75, 855. |
|
Suzuki, in Transition Metal Catalyzed |
9 |
S. Gronowitz, V. Bobosik, K. Lawitz, |
|
Reactions, A ‘Chemistry for the 21st |
|
Chem. Scripta 1984, 23, 120. |
|
Century’ monograph (Eds.: S. |
10 |
H. E. Katz, J. Org. Chem. 1987, 52, 3932. |
|
Murahashi, S. G. Davies), IUPAC, |
11 |
Y. Hoshino, N. Miyaura, A. Suzuki, |
|
Blackwell, Oxford, 1999, p. 441. (f ) A. |
|
Bull. Chem. Soc. Jpn. 1988, 61, 3008. |
|
Suzuki, J. Organomet. Chem. 1999, 576, |
12 |
R. S. Coleman, E. B. Grant, Tetrahedron |
|
147. |
|
Lett. 1993, 34, 2225. |
