
experment book
.pdf
Date : ...............................................................
Partners : ...............................................................
...............................................................
Does tap water contain dissolved gas? Grade : ...............................................................
PURPOSE : To show that gases dissolve in water, even though only slightly in most cases.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(1) |
• Wire gauze |
(1) |
• Tap water |
• Beaker, 500 mL |
(1) |
• Support rod |
(1) |
|
• Funnel |
(1) |
• Support base |
(1) |
|
• Thermometer |
(1) |
• Universal clamp |
(1) |
|
• Burner |
(1) |
• Bosshead |
(1) |
|
• Tripod |
(1) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Gases dissolve in water. Dissolution of gases in water is exothermic process. When an exothermic system is heated, the process is forced to go back. Thus, when water which
contains gas is heated the dissolved gas starts to escape from the water. It means that, solubility of gases in water decreases with increasing temperature.
PROCEDURE
Set-up |
Figure |
|
—Place the funnel with its discharge opening upwards in the beaker and fill the beaker about 4/5 full with tap water.
—Fill a test tube with water. Close the mouth of the tube with your thumb (or a piece of paper), then sink into the water in the beaker. Open the mouth of the tube.
Note: Be sure that water level is over the discharge opening of the funnel.
—Slip the test tube over the discharge opening of the funnel under the water surface.
Note: Use a funnel with short discharge tube.
—Sink the bulb of the temperature in the water and set the apparatus as seen in the Figure.
Note: Do not let the bulb of the thermometer touch the bottom of the beaker. It may exploit.
— Wear protective glasses.
Procedure
1.Heat the beaker with a small burner flame until the temperature of water reaches 30oC. Note your observations in “Observations and Data Tables”.
Experiment – 39 Does tap water contain dissolved gas? |
103 |
|

2.Extinguish the burner and wait about 5 minutes.
3.Measure the height of the collected gas in the tube. Record the measurement in the Table in “Observations and Data Tables”.
4.Refresh the tap water in the beaker. Set the apparatus as describe before.
5.Heat the beaker with a small burner flame until the temperature of water reaches 70oC.
6.Extinguish the burner and wait about 5 minutes.
7.Measure the height of the collected gas in the tube. Record the measurement in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental measurements.
Height of the collected gas at 30oC
Height of the collected gas at 70oC
................................ mm |
|
................................ mm |
|
|
|
EVALUATIONS AND CONCLUSIONS 


1.Draw conclusions from your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Which gases are probably dissolved in the tap water? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Give the reason that why the collected gas in the tube is not water vapour at all? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 39 Does tap water contain dissolved gas?
104

How can the electrical conductivity of a solution be arranged?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the effect of solute on the electrical conductivity of solution
To investigate the effect of the distance between electrodes on the electrical conductivity of a solution.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 250 mL |
(1) |
• Hydrochloric acid, 1M and 0.1M |
• Steel electrode |
(2) |
• Sodium sulphate, 1M |
• Power supply, 6V |
(1) |
• Ammonium hydroxide, 1M |
• Crocodile leads |
(3) |
• Acetic acid, 1M |
• Plug-in bulb mount |
(1) |
• Sugar, 1M |
• Bulb, 6V |
|
|
PRE-LAB DISCUSSION
Solution may conduct electrical current. Conductivity is accomplish ions in a solution between electrodes. Thus, if a solution does not contains positive and negative ions, it does not conduct electricity. The more ions in solution
means the more conductivity. Intensity of conductivity also depends on the distance between electrodes. The shorter the distance between electrodes, the higher the electrical conductivity in solutions.
PROCEDURE
Set-up
—Prepare 200 mL and 1M solutions of Hydrochloric acid, sodium sulphate, ammonium hydroxide, acetic acid and sugar.
Caution: Handle acids and bases with great care.
—Prepare 200 mL 0.1M Hydrochloric acid solution.
—Arrange the power supply for 6V.
—Place the electrodes at the maximum distance apart and parallel. Set the apparatus as seen in the Figure.
Procedure
1.Fill the beaker with 1M hydrochloric acid and switch on the 6V power supply. Note your observations on the intensity of bulb light in the Table in “Observations and Data Tables”.
2.Move the electrodes each other until the distance between electrodes becomes the half of the previous. Note your observations on the intensity of bulb light in the Table in “Observations and Data Tables”.
3.Switch off the power supply and discard the hydrochloric acid solution in the beaker and rinse the electrodes and beaker with distilled water. Re-assemble the apparatus.
4.Repeat the step 1 and 3 for the other solutions.
Note: Place the electrodes at the maximum distance apart and parallel for all the solutions.
Experiment – 40 How can the electrical conductivity of a solution be arranged? |
105 |
|

Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your observations on intensity of light as strong, bright and pale.
Solutions
Intensity of bulb light for the electrodes at maximum distance apart
Intensity of bulb light for the electrodes at half of the distance apart
1M HCl |
|
0.1M HCl |
|
1M Na2SO4 |
|
1M NH4OH |
|
1M HCH3COO |
|
|
|
|
|
|
|
|
|
.............. |
|
.............. |
|
.............. |
|
.............. |
|
.............. |
.............. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS 


1.Based on the experiment, what can be said about electrical conductivity of the solutions of the substances?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Which factors effects the electrical conductivity of the solutions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Define the terms below and classify the substances used in the experiment.
Strong electrolyte |
: ........................................................................................................................................................ |
Weak electrolyte |
: ........................................................................................................................................................ |
Non electrolyte |
: ........................................................................................................................................................ |
Experiment – 40 How can the electrical conductivity of a solution be arranged?
106

Do concentrations of ions in solutions affect the amount of precipitate?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To find the relative number of ions involved in precipitation reaction by measuring the height of the precipitate formed.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Test tube |
(5) |
• Potassium iodide solution, 0.5 M |
• Test tube rack |
(1) |
• Lead(II) nitrate solution, 0.5 M |
• Graduated cylinder, 10 mL |
(1) |
• Distilled water |
• Beaker, 100 mL |
(1) |
• Ruler |
• Stirring rod |
(1) |
|
PRE-LAB DISCUSSION
When two different solutions are mixed, there may be a ionexchange reaction between ions of the solutions. Precipitation reactions are the most common examples of such reactions. If there is a formation of solid as a result of mixing two solutions, this is called precipitation reaction. The
formed solid settles down. Because it is insoluble in water. The amount of ions which take role in precipitation, affects the quantity of the precipitate. The more ions mean the more precipitate. Thus quantity of precipitate is directly proportional to the quantity of ions.
PROCEDURE
Set-up
—Place five test tubes in the test tube rack and label them from 1 to 5.
—Place 5 mL potassium iodide solution into each test tube by using 10 mL graduated cylinder.
—Place about 15 mL lead(II) nitrate solution in 100 mL beaker.
Procedure
1.Add 1 mL lead(II) nitrate solution into the test tube-1,
1.5mL lead(II) nitrate solution into the test tube-2, 2 mL lead(II) nitrate solution into the test tube-3,
2.5mL lead(II) nitrate solution into the test tube-4, 3 mL lead(II) nitrate solution into the test tube-5.
2.Stir the solutions slowly, then let the solutions rest for about 5 minutes in the test tube rack. Note your observations in “Observations and Data Tables”.
Note: Stir the solutions equally.
3.When the precipitation is completed, measure the height of the each precipitate by ruler. Record the mea-
surements in the Table in “Observations and Data Tables”.
Figure
Experiment – 41 Do concentrations of ions in solutions affect the amount of precipitate? |
107 |
|
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental measurements.
Solutions |
|
|
|
|
|
Test tube-3 |
|
|
|
Test tube-5 |
|
Test tube-1 |
|
Test tube-2 |
|
|
Test tube-4 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
Volume of the potassium iodide solution |
|
|
|
mL |
|
mL |
|
|
|
mL |
|
mL |
|
|
|
mL |
|
||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Volume of the lead(II) nitrate solution |
|
mL |
|
mL |
|
mL |
|
mL |
|
mL |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Height of the precipitate |
|
mm |
|
|
|
mm |
|
|
|
mm |
|
|
mm |
|
|
mm |
|
||||
|
|
|
|
|
|
CALCULATIONS
2.Plot the graph of height of precipitate against the volume of lead(II) nitrate solution added.
EVALUATIONS AND CONCLUSIONS 


1.Based on the experiment, state a conclusion.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What can it be said about the ratio in which lead ion and iodide ion react? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the ionic and net ionic reaction equations for the experiment.
Ionic equation |
: ......................................................................................................................................................... |
Net ionic equation |
: ......................................................................................................................................................... |
4.What can be said about the concentration of the each ion in the solution after precipitation complete?
Test tube-1 : ..................................................................... |
Test tube-2 : ..................................................................... |
Test tube-3 : ..................................................................... |
Test tube-4 : ..................................................................... |
Test tube-5 : ..................................................................... |
|
Experiment – 41 Do concentrations of ions in solutions affect the amount of precipitate?
108

Some exothermic reactions in solutions
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine some exothermic reactions.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 250 mL |
(4) |
• Sulphuric acid, concentrated |
• Thermometer |
(1) |
• Hydrochloric acid, 2M |
• Graduated cylinder, 50 mL |
(1) |
• Sodium hydroxide solution, 2M |
• Balance |
(1) |
• Calcium chloride solution, 2M |
• Protective glasses |
(1) |
• Potassium carbonate solution, 2M |
|
|
• Ammonium nitrate |
PRE-LAB DISCUSSION
Chemical reactions are accompanied by a heat change. Two types of heat change occur. Those reactions which proceed with an evolution of heat to the surroundings are
called exothermic reactions. Those in which an absorption of heat from surroundings takes place in passing from reactants to products are called endothermic reactions.
PROCEDURE
Set-up |
Figure |
|
—Take four 250 mL beakers and label them from 1 to 4.
—Place the liquids as given below with graduated cylinder.
Beaker-1 : 100 mL distilled water Beaker-2 : 100 mL distilled water
Beaker-3 : 100 mL 2M sodium hydroxide solution Beaker-4 : 100 mL 2M calcium chloride solution
—Wait for several minutes to allow the temperature of the solutions become equal to the room temperature.
—Read the temperature of the solutions with thermometer and record the results in the Table in “Observations and Data Tables”.
—Wear protective glasses.
Procedure
1.Add 15 mL of concentrated sulphuric acid into the water in the beaker-1 and stir well with the thermometer.
Caution: Handle the concentrated acid with great care.
Note: While stirring the solutions be careful not to hit the bulb of the thermometer the beaker. It may be broken.
Experiment – 42 Some exothermic reactions |
109 |
|
2.Read the final temperature of the solution and record it in the Table in “Observations and Data Tables”.
3.Weigh about 10 g of ammonium nitrate crystals and add it to the water in the beaker-2 and stir well with the thermometer.
Note: Fine crystals dissolve more quickly.
Caution: It is not safe to grid ammonium nitrate in a mortar with a pestle.
4.Read the final temperature of the solution and record the it in the Table in “Observations and Data Tables”.
5.Add 100 mL 2M hydrochloric acid into the beaker-3 and stir well with the thermometer.
6.Read the final temperature of the solution and record in the Table in “Observations and Data Tables”.
7.Add 100 mL 2M potassium carbonate into the beaker- 4 and stir well with the thermometer.
8.Read the final temperature of the solution and record in the Table in “Observations and Data Tables.
OBSERVATIONS AND DATA TABLES
1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental readings.
Solutions in |
|
|
|
|
|
Beaker-3 |
|
Beaker-4 |
|
Beaker-1 |
|
Beaker-2 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Initial temperature |
|
....................... oC |
|
....................... oC |
|
....................... oC |
|
....................... oC |
|
|
|
|
|
|
|
|
|
Final temperature |
|
....................... oC |
|
....................... oC |
|
....................... oC |
|
....................... oC |
EVALUATIONS AND CONCLUSIONS 


1.Write the balanced reaction equations that take place in the beakers and classify them as endothermic and exothermic.
Beakers |
|
Reactions that take place in the beakers |
|
Exothermic or endothermic |
|
|
|
|
|
|
|
|
|
|
1 |
|
................................................. |
|
.......................................... |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
2 |
|
................................................. |
|
.......................................... |
|
|
|
||
|
|
|
|
|
3 |
|
................................................. |
|
.......................................... |
|
|
|
||
|
|
|
|
|
4 |
|
................................................. |
|
.......................................... |
|
|
|
||
|
|
|
|
|
2.List several changes in your environment and classify them as exothermic and endothermic.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Compare the energy changes that take place in chemical and physical changes.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 42 Some exothermic reactions
110
How can the heat of combustion of a liquid be determined?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the approximate value of the heat of combustion of ethyl alcohol.
EQUIPMENT and MATERIALS:
Equipment |
|
• Support rod |
(1) |
Chemicals and Other Materials |
• Beaker, 250 mL |
(4) |
• Universal clamp |
(1) |
• Tap water |
• Thermometer |
(1) |
• Bosshead |
(1) |
• Ethyl alcohol (ethanol) |
• Graduated cylinder, 50 mL |
(1) |
• Alcohol burner |
(1) |
|
• Balance |
(1) |
• Tripod |
(1) |
|
• Support base |
(1) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Energy change in a reaction is called heat of the reaction in general. Heats of special type of reactions have special names. For example, reaction heat of combustion is named as heat of combustion and reaction heat of neutralisation is
called as heat of neutralisation. In this experiment, molar heat of combustion of ethyl alcohol will be determined.
PROCEDURE
Set-up |
Figure |
—Weigh the 250 mL beaker and record the measurement in the Table in “Observations and Data Tables”.
—Place 200 mL tap water in the beaker with graduated cylinder.
—Place some ethyl alcohol (ethanol) in the alcohol burner, then weigh the burner. Record the result in the Table in “Observations and Data Tables”.
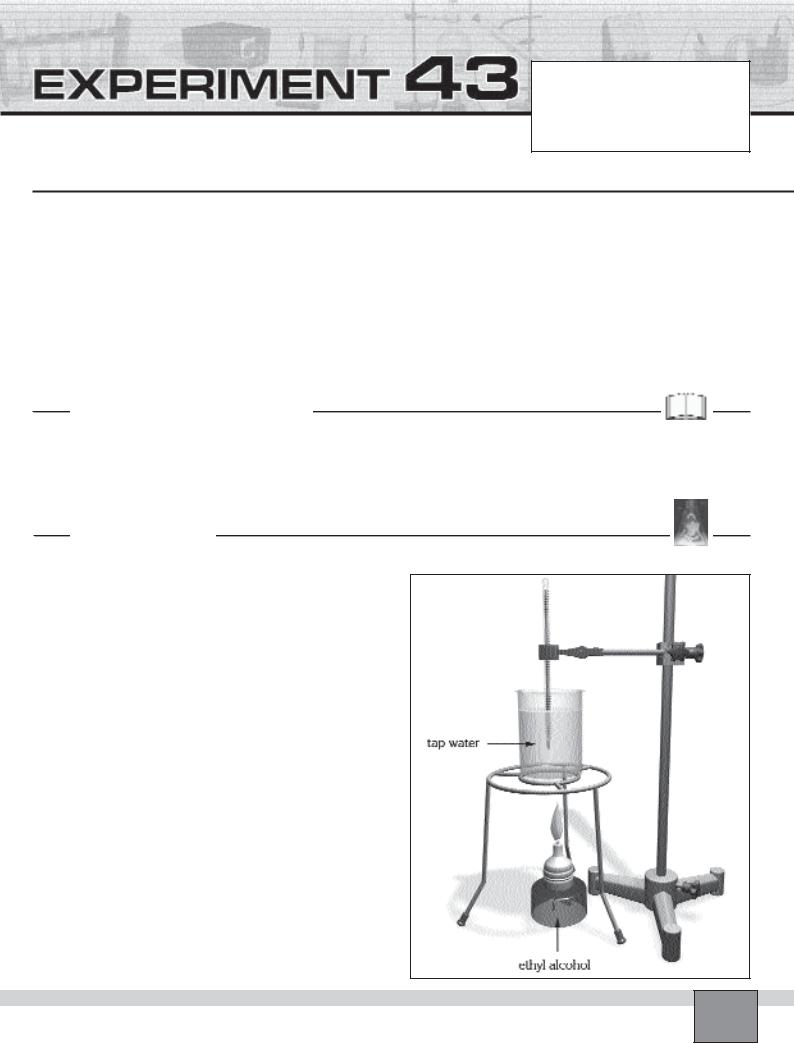
—Set the apparatus as seen in the Figure.
—Read the temperature of the water and record it in the Table in “Observations and Data Tables”.
Note: Wait for several minutes to allow the temperature of the water become equal to the room temperature.
— Wear protective glasses.
Procedure
1.Ignite the alcohol burner under the beaker and heat the water.
Note: Make sure that there is no air flow around the alcohol burner and the beaker.
— To protect the set from air flow, place an object which is not inflammable around the set as a shied.
Experiment – 43 How can the heat of combustion of a liquid be determined? |
111 |
|
2. Stir the water with thermometer at frequent intervals |
4. Re-weigh the alcohol burner with its content and record |
until the temperature has risen by about 30oC. |
in the Table in “Observations and Data Tables”. |
3.Extinguish the burner and read the temperature of the water after a thorough stirring. Record the reading in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES
1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental measurements.
|
|
Weight of beaker |
|
Weight of water |
|
Weight of Burner |
|
Temperature |
|
|
(WBeaker) |
|
(WWater) |
|
(WBurner) |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
At Initial |
|
................ g |
|
................ g |
|
................ g |
|
................ oC |
|
|
|
|
|
|
|
|
|
At Final |
|
- |
|
- |
|
................ g |
|
................ oC |
CALCULATIONS
1.Calculate the weight of the ethyl alcohol and temperature change as below
WEthanol = WBurner at initial – WBurner at final = |
............ |
– |
............ = ............ |
g |
|
T = TAt final – TAt initial = ............ |
– ............ |
= ............ |
|
°C |
|
2.Calculate the released heat by the burnt alcohol as below
QWater = mWater x cWater |
x T = ............ |
|
x 4.184 |
x |
............ = ............ |
J = ............ |
kJ |
|
QBeaker = mBeaker |
x cGlass |
x T = ............ |
|
x 0.84 |
x ............ |
= ............ |
J = ............ |
kJ |
QReleased = QWater |
x QBeaker = ............ |
+ |
............ = |
............ |
kJ |
|
|
|
3.Calculate the Molar heat of combustion of ethyl alcohol,
HMolar Heat of Combustion = MWEthanol |
QReleased ............ |
kJ |
|
x —————— = 46 g/mol x —————— = ................. |
kJ/mol |
||
|
WEthanol ............ |
g |
|
4. Calculate the error |
|
|
|
Theoretical value – Experimental value |
1264.3 – ............. |
|
% |
%Error = ————————————————————— = ——————————— = ................. |
|||
Theoretical value |
1234.3 |
|
|
Experiment – 43 How can the heat of combustion of a liquid be determined?
112
