
experment book
.pdf
Note: Be sure that there are no bubbles in the tip. To do this open |
Figure |
the stopcock of the buret with your left hand and let the solution |
|
|
|
go down and fill the tip of the buret. Close the stopcock. |
|
—Read and record the volume of hydrochloric acid as initial volume in the Table in “Observations and Data Tables”.
Note: Read the volume of the solution as described at the beginning of the book.
—Place a white paper on the bench under the erlenmeyer flask as seen in the Figure.
Procedure
1.Place 20 mL of sodium hydroxide solution into the 250 mL erlenmeyer flask.
2.Add three drops of phenolphthalein indicator. Swirl the erlenmeyer flask to mix its contents.
3.Hold the erlenmeyer flask with your right hand and swirling it gently.
4.Meanwhile open the stopcock of the buret so that hydrochloric acid drops onto the sodium hydroxide solution slowly.
Note: Do not stop swirling.
5.Continue addition of hydrochloric acid until the permanent colour change occurs.
Note: Be careful that permanent colour change occurs after addition of one drop at the endpoint.
6.Close the stopcock of the buret read and record the final volume of the remaining hydrochloric acid in the
buret in the Table in “Observations and Data Tables”.
7.Repeat the procedure two times more and record the measurements in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES
1.Note your observations
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your measurements in the experiment.
Trials |
|
Initial volume of the |
|
Final volume of the |
|
Used hydrochloric acid |
|
|
hydrochloric |
acid (Vi) |
|
hydrochloric acid (Vf) |
|
( V=Vi- Vf) |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
First trial |
|
....................... |
mL |
|
....................... mL |
|
....................... mL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second trial |
|
....................... |
mL |
|
....................... mL |
|
....................... mL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third trial |
|
....................... |
mL |
|
....................... mL |
|
....................... mL |
|
|
|
|
|
|
|
|
Experiment – 54 How can you determine the concentration of an unknown base solution? |
143 |
|

CALCULATIONS
|
|
|
|
|
|
|
MHCl |
x VHCl |
||
1. For each trial, calculate the molarity of the NaOH solution by using the expression MNaOH = ——————— and record |
||||||||||
the Table. |
|
|
|
|
|
|
VNaOH |
|||
|
|
|
|
|
|
|
|
|
||
|
|
0.1 x ................. |
|
|
|
|
|
|
|
|
For trail - 1 : MNaOH = ——————————— = ............. |
|
mol/L |
|
|
|
|
|
|||
|
|
........................... |
|
|
|
|
|
|
|
|
|
|
0.1 x ................. |
|
|
|
|
|
|
|
|
For trail - 2 : MNaOH = ——————————— = ............. |
|
mol/L |
|
|
|
|
|
|||
|
|
........................... |
|
|
|
|
|
|
|
|
|
|
0.1 x ................. |
|
|
|
|
|
|
|
|
For trail - 3 : MNaOH = ——————————— = ............. |
|
mol/L |
|
|
|
|
|
|||
|
|
........................... |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trial - 1 |
|
Trial - 2 |
|
Trial - 3 |
|
|
Average |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration of NaOH |
|
mol/L |
|
mol/L |
|
mol/L |
|
|
mol/L |
|
|
|
|
|
|
|
||||
EVALUATIONS AND CONCLUSIONS 


1.Define the following terms;
a) Standard solution |
: ................................................................................................................................................. |
b) Titration |
: ................................................................................................................................................. |
c) End point |
: ................................................................................................................................................. |
d) Indicator |
: ................................................................................................................................................. |
2.Compare your results with other friends. Explain the differences.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.If 30 mL of 0.5 M KOH is needed to neutralise unknown 10 mL HCl solution, what is the molarity of the HCl solution?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.List other types of indicators and their pH range.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.List some usages of the titration in different area?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 54 How can you determine the concentration of an unknown base solution?
144
How do metals behave in salt solutions? (activity of metals)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe the behaviour of metals in salt solutions.
To observe that noble metals can be obtained from their salt solutions by the help of metals.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Zinc nitrate, 1M |
• Test tube, |
(4) |
• Iron nails |
• Aluminium nitrate, 1M |
• Test tube rack |
(1) |
• Zinc pieces |
• Copper(II) nitrate, 1M |
|
|
• Aluminium pieces |
• Silver nitrate, 1M |
|
|
• Copper pieces |
• Emery paper |
|
|
• Iron(II) nitrate, 1 M |
• Marker pen |
PRE-LAB DISCUSSION
It is a fact that certain metals will displace other metals in water solutions of their salts . Then, metals can be arranged in a series such that any metal higher up in the series will displace from its salts any metal below it. The greater the
gap separating the metals in the series, the more readily does displacement take place. The series gives us a good estimate of the chemical activity of the metal itself.
PROCEDURE
Set-up |
Figure |
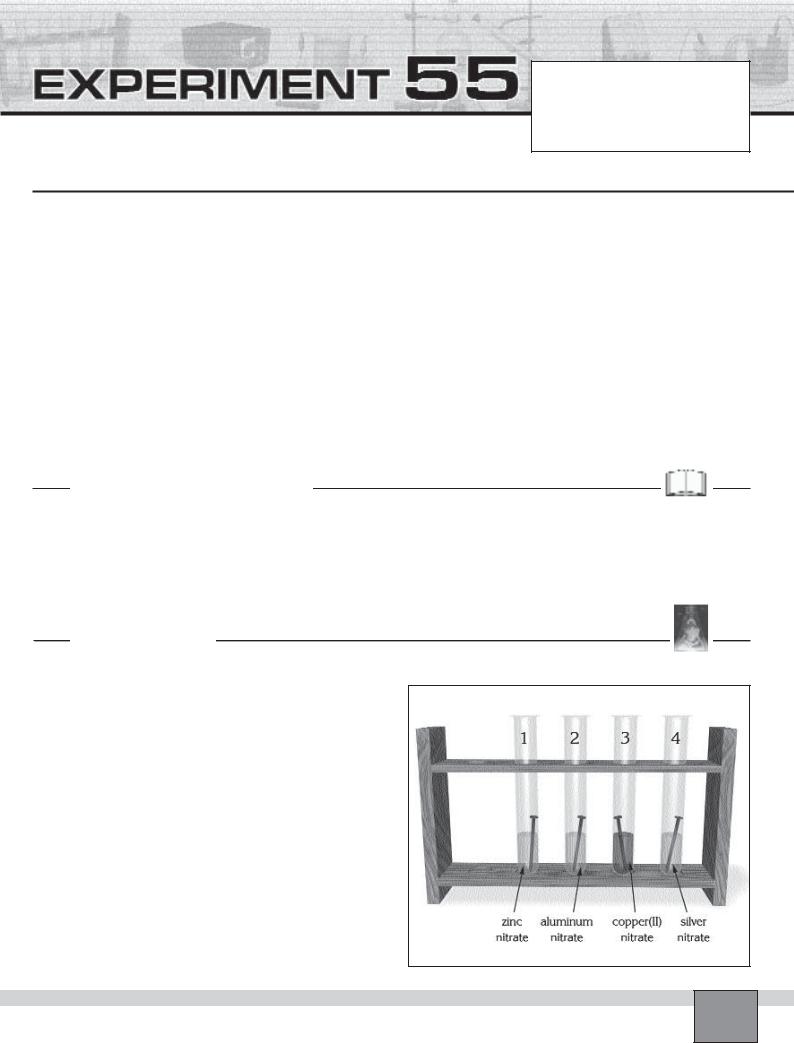
—Place five test tubes in the test tube rack and number them from 1 to 5.
—Clean the surface of the iron nail, zinc, aluminium and copper metals with emery paper to get shiny surface.
Note: Steel nail is not suitable for the experiment.
Procedure
1.Fill the one fourth of the
—Test tube-1 with zinc nitrate
—Test tube-2 with aluminium nitrate
—Test tube-3 with copper(II) nitrate
—Test tube-4 with silver nitrate
Caution: Do not swallow salts and their solutions. Because they are hazardous for health.
2.Put a clean iron nail into the each solution in the test tubes.
Experiment – 55 How do metals behave in salt solutions? (activity of metals) |
145 |
|
3.Wait about 1 minute and observe the solutions for changes. Record your observations in the Table in “Observations and Data Tables”.
4.Repeat the steps from 1 to 3 for the other metals.
Note: Use the solutions which do not contain ions of the metal being test.
OBSERVATIONS AND DATA TABLES 


1.Note your observations in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2. Place |
“x” in the table where a reaction |
takes place and |
|
“-” for the non reacting pairs. |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Metal |
|
|
Fe+2 |
|
|
Zn+2 |
|
Al+3 |
|
Cu+2 |
|
Ag+ |
Iron nail
Zinc
Aluminium 

Copper
EVALUATIONS AND CONCLUSIONS
1.Set a reactivity order for the metals on the basis of the table in the observations and data tables.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write a reaction equation for each process that has taken place in the experiment.
.............................................................................. ..............................................................................
.............................................................................. ..............................................................................
.............................................................................. ..............................................................................
.............................................................................. ..............................................................................
.............................................................................. ..............................................................................
3.State which property of the metals is actually described with reactivity order?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What does the change in the colour of the solutions tell us?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 55 How do metals behave in salt solutions? (activity of metals)
146
Where does carbon come in the reactivity series? (extraction of metals)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe the reactivity of carbon among some metals.
To observe the extraction of some metals from its oxides.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Crucible |
(1) |
• Magnet |
(1) |
• Lead(II) oxide |
• Tongs |
(1) |
• Protective glasses |
(1) |
• Iron(III) oxide |
• Burner |
(1) |
|
|
• Copper(II) oxide |
• Tripod |
(1) |
|
|
• Carbon, (Powdered wood charcoal) |
• Clay triangle |
(1) |
|
|
• White paper sheet |
PRE-LAB DISCUSSION
The reactivity series of the metals indicates, in general, the inverse order in which the elements are isolated. Thus, metals low in the series such as gold, silver, and lead have been known since very early times. Metals which are high in the series, were proved very difficult to isolate. Metals which are not found as the free elements in nature, can be obtained
from ores such as oxides and salts. This is called extraction. During extraction, the metallic ion takes up the necessary number of electrons to convert it to the corresponding atom.
The electrons are supplied by reducing agent such as carbon.
PROCEDURE
Set-up |
Figure |
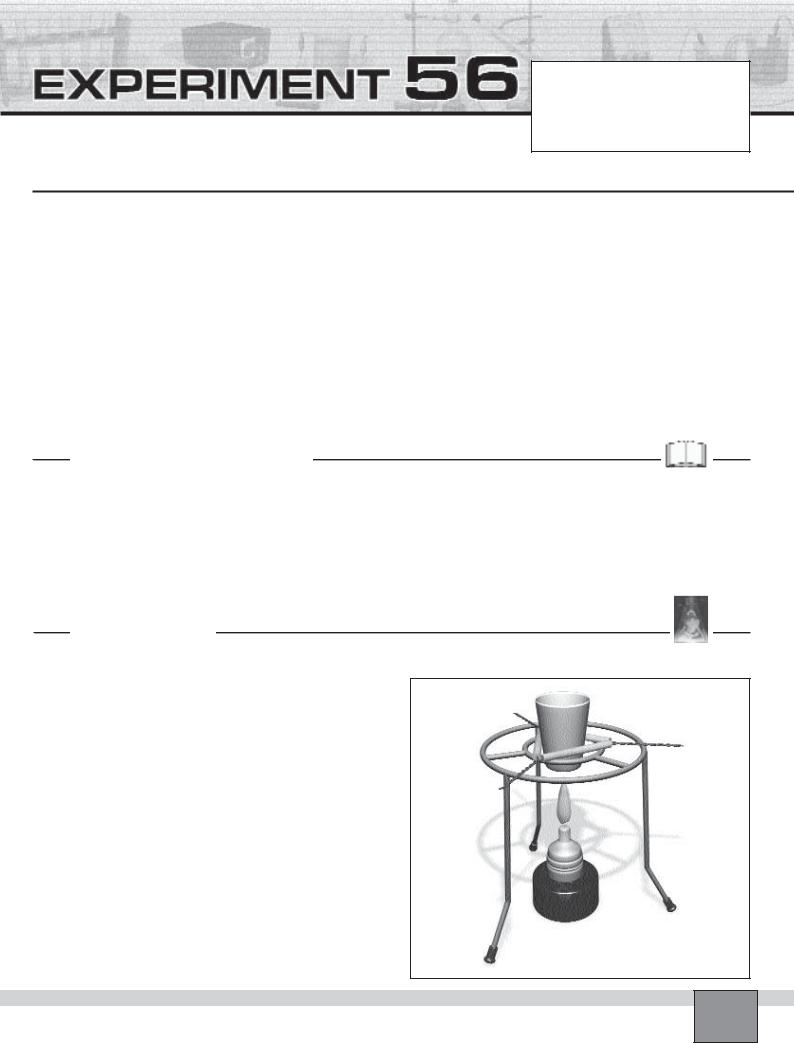
—Put a spatula of lead(II) oxide and a spatula of carbon in a crucible.
Note: Powdered wood charcoal is suitable for carbon.
—Mix the content of the crucible.
—Set the apparatus as seen in the Figure.
—Wear protective glasses.
Procedure
1.Ignite the burner and heat the crucible strongly until a beads of molten metal forms. Note your observations in “Observations and Data Tables”.
Caution: Do not handle the beads, they may be hot.
2.Extinguish the burner and let the beads to cool.
3.Wash the beads and rub one of them on a white paper with the fingernail. Note your observations in “Observations and Data Tables”.
Experiment – 56 Where does carbon come in the reactivity series? (extraction of metals) |
147 |
|

4.Proceed the experiment by using iron(III) oxide and Carbon.
5.To test iron, hold a magnet near the extracted iron ingot. Note your observations in “Observations and Data Tables”.
Caution: Do not handle the ingot before cooling it.
6.Proceed the experiment by using copper(II) oxide and Carbon. Record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations for lead(II) oxide and carbon mixture.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations for iron(IlI) oxide and carbon mixture.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Note your observations for copper(lI) oxide and carbon mixture.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Set placement of carbon in the reactivity order among the used metal.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write a reaction equation for each process that has taken place.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.How are metals extracted in industry? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Are all the metals extracted by using carbon? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 56 Where does carbon come in the reactivity series? (extraction of metals)
148
Several oxidation - reduction reactions in solutions
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the nature of the Redox reactions.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Test tubes |
(2) |
• Potassium permanganate, 0.05 M |
• Test tube rack |
(1) |
• Potassium dichromate, 0.02 M |
• Dropper |
(1) |
• Iron(II) sulphate, 0.5 M |
• Marker pan |
|
• Sulphuric acid, concentrated |
PRE-LAB DISCUSSION
Substances that lose electrons during a chemical reaction are said to be “oxidised”. Those that gain electrons are said to be “reduced.” If one reactant gains electrons, another must lose an equal number of electrons. Thus, oxidation and reduction must occur simultaneously to a comparable degree. The relative strengths of oxidation and reducing agents can be determined experimentally.
Many chemical reactions involve (or at least appear to involve) a shift of electron density from one atom to another. Collectively, such reactions are called oxidation-reduction reactions or simply redox reactions. The term oxidation refers to the
loss of electrons (or electron density) by another. For example, the reaction between sodium and chlorine involves a loss of electron by sodium (oxidation of sodium) and a gain of electron by chlorine (reduction of chlorine). We can write these changes in equation from including the electrons
Na |
Na+ + e– |
(oxidation) |
|
Cl |
2 |
+ 2e– 2Cl– |
(reduction) |
|
|
|
|
We say that sodium is oxidised and chlorine is reduced where as sodium is reducing agent, chlorine is oxidising agent.
PROCEDURE
Set up |
Figure |
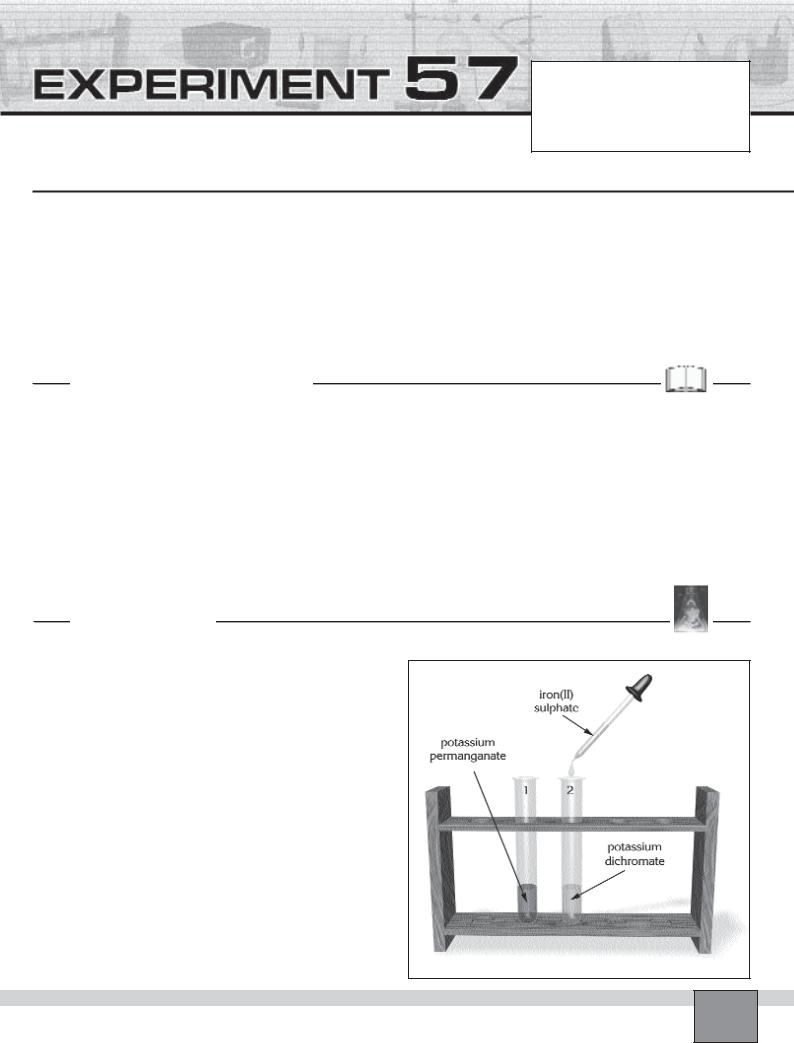
—Place two test tubes in the test tube rack and number them.
—Put about 5 mL of 0.05 M potassium permanganate solution into the test tube 1. Then add 10 or 15 drops of concentrated sulphuric acid with a dropper.
Caution: Always handle acids with great care.
—Put about 5 mL of 0.02 M potassium dichromate solution into the test tube 2. Then add 10 or 15 drops of concentrated sulphuric acid with a dropper.
Procedure
1.Add 0.5 M iron(II) sulphate solution to the test tube 1 drop by drop until the purple colour just disappears. Record your observations in “Observations and Data Tables”.
2.Add 0.5 M iron(II) sulphate solution to the test tube 2 drop by drop until the orange colour turns to green. Record your observations in “Observations and Data Tables”.
Experiment – 57 Several oxidation - reduction reactions in solutions |
149 |
|

OBSERVATIONS AND DATA TABLES 


1.Note your observations and colour change for potassium permanganate.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations and colour change for potassium dichromate.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Define the following terms.
Oxidising agent : ..............................................................................................................................................................
Reducing agent : ..............................................................................................................................................................
2.Write the chemical reactions and indicate the oxidised and reduced elements, and oxidising, reducing agents for each process.
In the first reaction |
: ................................................................................................................................................. |
|
................................................................................................................................................. |
In the second reaction |
: ................................................................................................................................................. |
|
................................................................................................................................................. |
3.How can be deduced that there is an oxidation and reduction reaction occur in the solution? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 57 Several oxidation - reduction reactions in solutions
150
How can an iron nail be protected from rusting?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To protect metals by coating another metal.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 150 mL |
(3) |
• Copper(II) sulphate, 1M |
• Tongs |
(1) |
• Potassium hexacyanoferrate, 0.2M |
• Emery paper |
(1) |
• Filter paper |
• Protective glasses |
(1) |
• Rubber gloves |
• Iron nail |
(2) |
|
PRE-LAB DISCUSSION
Many metals, especially iron, undergo corrosion when expose to air and water. Many methods and devices have ben developed to prevent or retard corrosion. Iron can be protected against corrosion by coating it with an organic material such as paint and grease; with another metal such as
zinc, copper, tin. Some alloys of iron are corrosion - resistant such as steel. Cathodic protection which is application of electrochemistry may be used to prevent the corrosion of iron.
PROCEDURE
Set-up
—Clean 2 iron nails with emery paper until they have continuous shining surface.
—Put on rubber gloves.
—Put three beakers on the bench and number them from 1 and 3.
—Fill the 2 cm height of the beaker 1 with copper(II) sulphate solution.
—Fill the 2 cm height of the beaker 2 and 3 with potassium hexacyanoferrate solution.
Caution: Do not swallow the salt solution and avoid to skin contact.
— Wear protective glasses.
Procedure
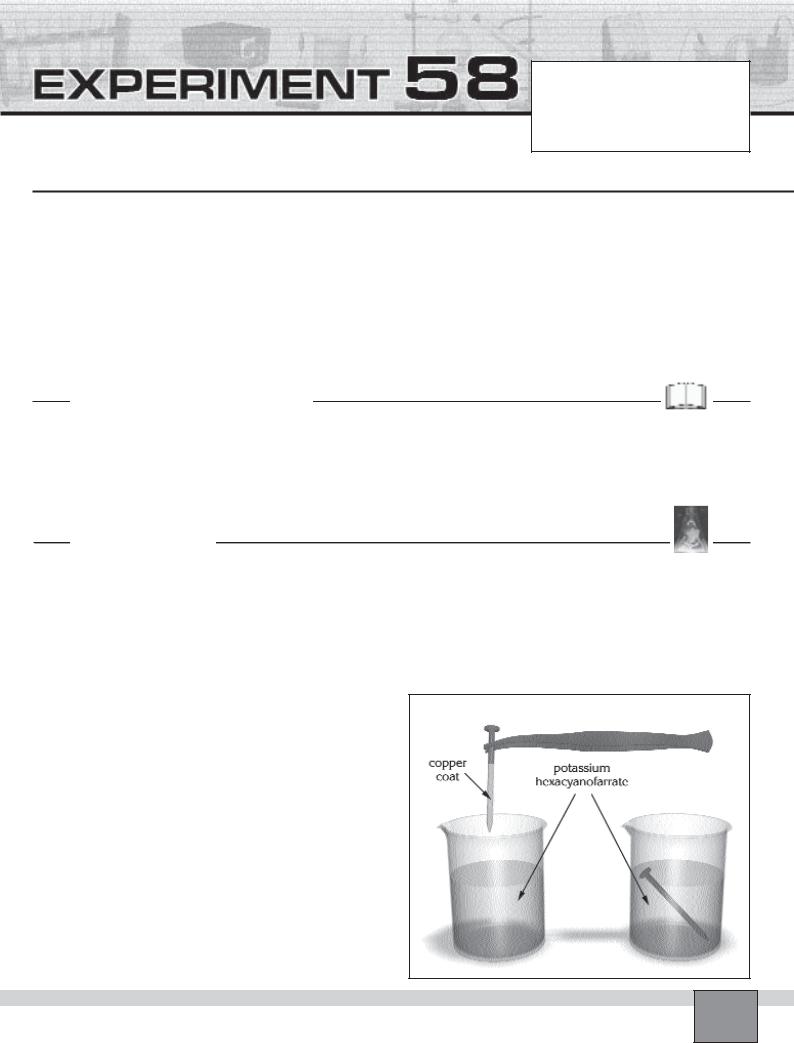
1.Place one of the nails into the copper(II) sulphate solution with tongs.
2.Wait until the nail has just become covered with a copper layer.
3.Take the covered nail out from the solution with the tongs, then dry it with filter paper without removing the copper layer.
4.Place the covered nail into the potassium hexacyanoferrate solution in the beaker 2 and the second nail in-
to the other potassium hexacyanoferrate solution.
5.Wait for about 5 minutes and observe the colours of the solutions in the beakers. Record your observations in “Observations and Data Tables”.
Note: Blue colour of hexacyanoferrate solution indicates the formation of iron ion ( corrosion)
Figure
Experiment – 58 How can an iron nail be protected from rusting? |
151 |
|

OBSERVATIONS AND DATA TABLES 


1.Note your observations in the table below.
Colour of the potassium haxacyanoferrate solution
|
|
Before |
|
After |
|
|
|
|
|
|
|
|
|
|
Nail with copper coat |
|
................................... |
|
................................... |
|
|
|
|
|
|
|
|
||
Nail without copper coat |
|
................................... |
|
................................... |
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS
1.Draw the conclusions from the observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the role of the copper layer in the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What other metals can be used instead of copper to protect iron from corrosion?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Give several methods to protect metals from corrosion with examples.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 58 How can an iron nail be protected from rusting?
152
