
experment book
.pdf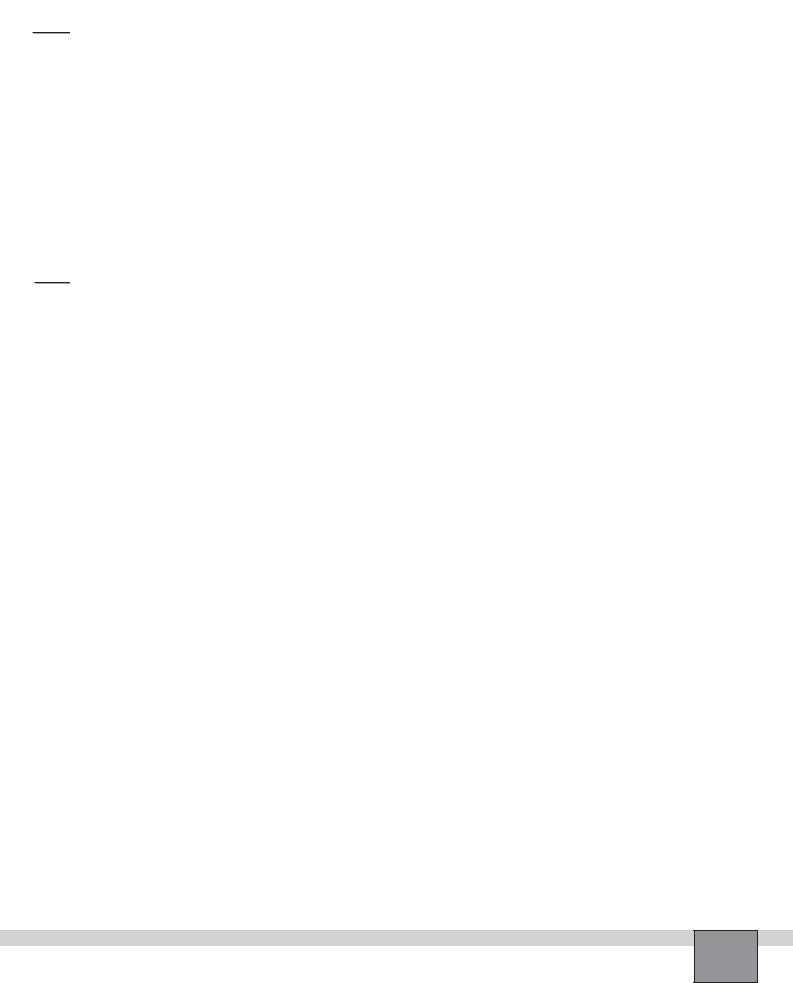
OBSERVATIONS AND DATA TABLES 


1.Note your observations on heating the metals in the crucible.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on molten metal.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of metals is used to separated iron and tin?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How this method is used in industry? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Can all metal mixtures be separated by this method? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 15 Can melting point difference be used to separate metal mixtures? |
43 |
|
Chromatography
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe the separation of pigments by chromatography.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 100 mL |
(1) |
• Acetone, (or ethanol) |
• Dropper |
(1) |
• Grass (or spinach) |
• Mortar with pestle |
(1) |
• Circular filter paper |
• Test tube |
(1) |
|
PRE-LAB DISCUSSION
Pigments which appear to be uniform, even black, can consist of several differently coloured components. Adhesion of the pigment components on the paper fibres is different.
The solvent transports the pigments which have different adhesiveness away from the centre at different speeds.
PROCEDURE
Set-Up |
Figure |
—Cut up a handful of grass (or spinach) into small pieces with scissors.
—Grind the pieces with enough acetone (or ethanol) to produce 2-3 mL of free liquid in a mortar with pestle. Then pour the liquid in a test tube.
Note: The extract should be as concentrated as possible.
Caution: Acetone and ethanol are highly inflammable
Procedure
1.Place a circular filter paper on a 100 mL beaker. Using a dropper place a drop of extract in the centre of the paper.
2.Let the liquid spread, then add another drop and repeat until 3-4 drops have been added.
3.One at a time, add more drops of acetone (or ethanol). Observe the colour bands on the paper. Record your observation in “Observations and Data Tables”.
Experiment – 16 Chromatography
44

OBSERVATIONS AND DATA TABLES 


1.Note your observations in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of matter is used in the chromatography?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the function of the solvent in the experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.In which area of industry chromatography is used? Research and give examples.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 16 Chromatography |
45 |
|
|
|
Date |
...............................................................: |
|
|
|
Partners : ............................................................... |
|
|
|
|
|
............................................................... |
|
|
Can a compound be decomposed into |
|
|
|
|
Grade |
: ............................................................... |
|
|
|
new substances by electricity? (Electrolysis) |
|
|
|
|
|
|
|
|
PURPOSE : To decompose water into the simpler substances by means of electricity.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Beaker, 500 mL |
(1) |
• Steel electrode |
(2) |
• Sodium carbonate |
• Test tube |
(2) |
• Pieces of wires, 30-40 cm |
(2) |
• Distilled water |
• Stirring rod |
(1) |
• Crocodiles |
(4) |
• Wood splint |
• Power supply |
(1) |
|
|
• Ruler |
PRE-LAB DISCUSSION
Electrical energy is easy to produce, and easy to transport from one place to another. It does not cause any environmental pollution either. Therefore it is frequently used in industry in the extraction of many metals from their ores, and in the decomposition of many compounds into their constituent substances.
In electrolysis, the electricity is applied to the liquid by means of the metals immersed into the water. These metals
are called electrodes. The substance that will be decomposed is called electrolyte.
Water can be decomposed into oxygen and hydrogen gases by electricity passing through it.
PROCEDURE
Set-up
—Fill the beaker about 2/3 full of distilled water.
—Fill one of the test tubes (or graduated cylinder) with water. Close the mouth of the tube with your thumb (or a piece of paper), then sink into the water in the beaker. Open the mouth of the tube. Apply the same process for the second test tube.
—Set up the apparatus as in the Figure.
Procedure
1.Apply a voltage of between 5 and 10 V. Observe if there is any change occurs on the surface of the electrodes.
2.Add a few crystals of sodium carbonate into the water and dissolve it. Observe the rate of formation of gas bubbles on the electrodes. Note your observations in “Observations and Data Tables”.
3. Add more sodium carbonate with a spatula into the water and dissolve it. Observe gas bubbling rate on the
electrodes. Note your observations in “Observations and Data Tables”.
4.When sufficient gases are collected in the tubes, switch off the power supply. Measure the height of the gases in each tube. Record the result in “Observations and Data Tables”.
5.Perform the hydrogen test with the gas collected at the cathode (+) .
Hydrogen test : Close the opening of the tube with your thumb. Move the test tube toward the flame, remove your thumb and place the opening of the tube close the flame.
6.Perform the smouldering splint test with the gas collected at the anode (-).
Smouldering test: Close the opening of the tube with your thumb. Ignite a splint. Then blow the flame out. Insert the smouldering end of the splint into the tube.
Experiment – 17 Can a compound be decomposed into new substances by electricity? (Electrolysis)
46

Figure
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OBSERVATIONS AND DATA TABLES |
|
|
|
|
|
|||||
1. |
|
|
|
|
|
|
|
||||||
Note your observation before addition of sodium carbonate on the electrodes. |
|
|
|
|
|||||||||
|
|
|
|
||||||||||
|
........................................................................................................................................................................................... |
||||||||||||
|
........................................................................................................................................................................................... |
||||||||||||
2. |
Note your observation after addition of sodium carbonate on the electrodes. |
|
|
|
|
||||||||
|
........................................................................................................................................................................................... |
||||||||||||
|
........................................................................................................................................................................................... |
||||||||||||
3. |
Fill the table below with your measurements. |
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Hydrogen |
|
|
Oxygen |
|
H2 / O2 ratio |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Height |
|
|
cm |
|
|
cm |
|
/ |
|
||
|
|
|
|
|
|
|
....................... |
|
|
||||
4. |
Record the result of the hydrogen and smouldering tests. |
|
|
|
|
|
|||||||
|
a) Hydrogen test |
: ........................................................................................................................................................ |
|
|
|
|
|
|
|
|
|
||
|
b) Smouldering test |
: ........................................................................................................................................................ |
|
|
|
|
|
|
|
|
|
||
EVALUATIONS AND CONCLUSIONS
1.Why did you observe an increase in gas evolution on the electrodes with addition of sodium carbonate?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What does the H2/O2 ration tell us? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 17 Can a compound be decomposed into new substances by electricity? (Electrolysis) |
47 |
|

3.Compare the some properties of oxygen, hydrogen and water.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Can any salt be used instead of Na2CO3? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.How do the following affect the rate of electrolysis of water.
a.The quantity of water electrolysed.
.....................................................................................................................................................................................
.....................................................................................................................................................................................
b.The quantity of sodium carbonate dissolved in water?
.....................................................................................................................................................................................
.....................................................................................................................................................................................
c.The applied voltage.
.....................................................................................................................................................................................
.....................................................................................................................................................................................
Experiment – 17 Can a compound be decomposed into new substances by electricity? (Electrolysis)
48
Can a compound be decomposed by heating?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe that a compound can be decomposed thermally.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Crystallising dish |
(1) |
• Support rod |
(2) |
• Mercury (II) oxide |
• Test tube |
(3) |
• Universal clamp |
(3) |
• Wood splint |
• Right-angled glass tube |
(1) |
• Boss head |
(3) |
|
• Delivery glass tube |
(1) |
• Spatula |
(1) |
|
• Burner |
(1) |
• Rubber tubing, 10 cm |
(1) |
|
• Support base |
(2) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Compounds can be decomposed into their constituent elements only by chemical changes. In a chemical change, different substances are produced from original substances.
When some salts are heated, they undergo a chemical change and decompose. As a rule, this already happens at lower temperatures when gaseous decomposition products appear.
PROCEDURE
Set-up
—Place 2/3 of crystallising dish with water. Then fill two or three test tubes with water and invert them into the water in crystallising dish.
—Place a spatula of mercury(II) oxide in an another test tube. Set the apparatus as seen in the Figure.
Note: Lubricate the glass-rubber connection with glycerol.
— Wear protective glasses.
Procedure
1.Heat the mercury(II) oxide gently with a low non-lumi- nous burner flame. Observe the change taking place in the tube. Record your observation in “Observations and Data Tables”.
Caution: Take care to heat only the lowest part of the test tube. Do not heat the drops of mercury because mercury vapour is very poisonous.
2.When the first gas collecting tube is full of gas, shift the
lower end of the delivery tube to the other inverted test tube.
Note: Do not take the test tube out from the water.
3.When no more gas is evolved, stop heating.
Note: The delivery tube should be removed from water before heating is stopped. Otherwise water may be forced into the hot test tube.
4.Do smouldering test (oxygen test) for the collected gas in the test tubes. Record your observation in “Observations and Data Tables”.
Smouldering test: Close the opening of the tube with your thumb and take the test tube out. Ignite a splint. Then blow the flame out. Immediately, remove your thumb and Insert the smouldering end of the splint into the tube.
Caution: After examining the mercury, it may be returned to the laboratory stock of impure mercury.
Experiment – 18 Can a compound be decomposed by heating? |
49 |
|

Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations on the mercury(II) oxide and surface of the test tube.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on smouldering test of collected gas.
..................................................................................................................................................................................
..................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the chemical reaction equation for the experiment.
...........................................................................................................................................................................................
2.Compare the properties of the mercury(II) oxide and the products.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Can all compounds be decomposed by heating as this experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Compare the decomposition of a compound with separation of a mixture.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 18 Can a compound be decomposed by heating?
50
Do salts generate light?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe the characteristic colours produced by certain metal ions when heated in flame. Identify unknown metal ions by means of flame test.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(1) |
• Graduated cylinder, 10 mL |
(1) |
• Salts of sodium, potassium, lithium, |
• Watch glass |
(7) |
• Burner |
(1) |
calcium, barium and copper. |
• Beaker, 100 mL |
(1) |
• Nichrome wire loop, (or platinum) |
(1) |
• Hydrochloric acid, concentrated. |
PRE-LAB DISCUSSION
The normal electron configuration of atoms or ions of an element is known as the “ground state.” In this most stable energy state, all electrons are in the lowest energy level available. When atoms or ions in the ground state are heated to high temperatures, some electrons may absorb enough energy to allow them to “jump” to higher energy levels. The atom is then said to be in the “excited state”. This excited electron is unstable and it comes back to its original lower energy level. The absorbed energy is emitted in the form of electromagnetic energy some of this energy may be in the form of visible light. The colour of this light can be used to identify the elements. Because different metal ions produce different colours in flame test. This method is known as flame test.
Only metals, with their loosely held electrons are excited in the flame. Thus, flame test is useful in identification of met-
al ions. Many metal ions exhibit characteristic colours when vapourised in the flame. In this experiment, characteristic colours of several different metal ions will be observed and an ion will be identified by means of its flame test.
Sodium salts, for example, give a bright yellow flame. If you have ever softened a glass rod or glass tubing in a bunsen burner flame, you have seen this yellow colour; it is produced by sodium ions that are vapourised from the hot glass.
If a sample is suspected to contain sodium, a clean wire is dipped into a solution of the sample and then held in a flame. A bright yellow colour in the flame confirms the presence of sodium. The absence of the bright flame means that the sample does not contain sodium.
PROCEDURE
Set-up
—Place a little amount of salts of sodium, potassium, lithium, calcium, barium and copper on watch glasses (or pieces of paper) and label them. Take concentrated hydrochloric acid in a 10 mL graduated cylinder.
Caution: Be careful in handling concentrated acid solutions.
Procedure
1.Adjust the burner flame for non-luminous flame. Clean the nichrome wire by dipping it into the hydrochloric acid solution and heat it to redness.
2.Take a few crystals of sodium nitrate salt on nichrome
wire loop, and then hold the wire in a non-luminous burner flame.
3.Observe the colour of the flame and note your observation in the Table in “Observations and Data Tables”.
4.Clean the nichrome wire loop as in the step-1 for each flame test.
Note: Use fresh hydrochloric acid for each test.
5.Repeat above steps for other metal salts and record the colour of the flame in the Table in “Observations and Data Tables”.
6.Perform a flame test for an unknown sample given to you and try to determine the metal ion in it.
Experiment – 19 Do salts generate light? |
51 |
|
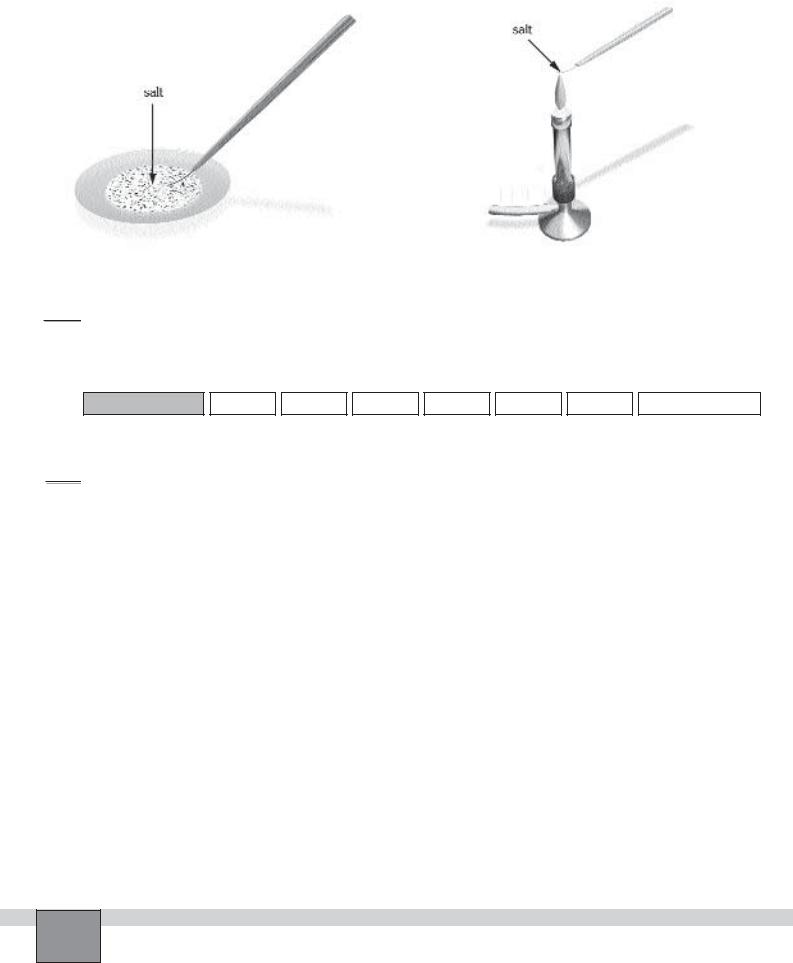
Figure-1 |
|
Figure-2 |
|
|
|
|
|
|
OBSERVATIONS AND DATA TABLES 


Complete the following tables
Metallic Ion |
|
Na+ |
|
Ka+ |
|
Li+ |
|
Ca+2 |
|
Ba+2 |
|
Cu+2 |
|
Unknown (.....) |
Flame Colour
EVALUATIONS AND CONCLUSIONS 


1.Explain how the colours in the flame test are produced?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Define the terms below;
a) Ground state : .........................................................................................................................................................
...........................................................................................................................................................................................
b) Excited state : .........................................................................................................................................................
...........................................................................................................................................................................................
3.Which of the elements produce the similar colour in the flame test? Why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.If NaNO3 and Cu(NO3)2 were mixed what kind of colour would you expect in the flame test? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Can the flame test be used to distinguish metals in a sample? Explain.
...........................................................................................................................................................................................
Experiment – 19 Do salts generate light?
52
