
experment book
.pdf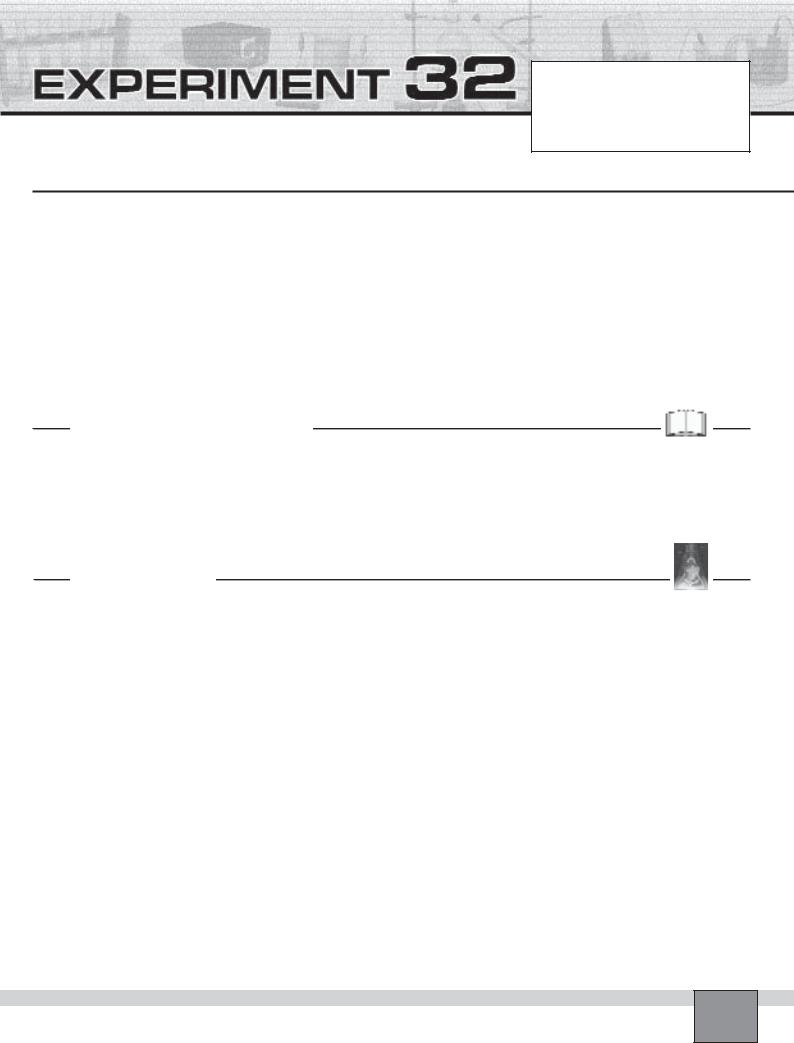
How can molar volume of a gas be determined?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the molar volume of a gas at room and at standard conditions.
EQUIPMENT and MATERIALS:
Equipment |
|
• Thermometer |
(1) |
• Protective glasses |
(1) |
• Beaker, 500 mL |
(1) |
• Rubber tubing, 20 cm |
(1) |
|
|
• Gas measuring tube, 50 mL |
(1) |
• Rubber stopper, with two holes |
(1) |
Chemicals and Other materials |
|
• Erlenmeyer flask |
(1) |
• Support base |
(2) |
• Sodium carbonate |
|
• Separatory funnel |
(1) |
• Support rod |
(2) |
• Hydrochloric acid, 3M |
|
• Right - angled glass tube |
(1) |
• Universal clamp |
(2) |
• Distilled water |
|
• Delivery glass tube |
(1) |
• Bosshead |
(2) |
|
|
PRE-LAB DISCUSSION
According to the Avogadro’s principle the molar volume of all gases is equal at the same pressure and temperature. Based on this, scientists found that the volume of 1 mol of a gas is 22.4 L at standard pressure and temperature (STP). Standard pressure and temperature are 1 atm and 273 K (0 °C). When the standard conditions are changed, the vol-
ume of gases will also change. Change in volume can be found by the ideal gas equation. (PV = nRT)
P = pressure |
V = volume |
n = mol |
R = gas constant |
T = temperature in K |
|
PROCEDURE
Set-up
—Put two spatula of sodium carbonate into the erlenmeyer flask.
—Insert the separatory funnel into one of the rubber stopper’s holes and the right-angled glass tube into the other hole.
Note: Lubricate glass-rubber connections with glycerol before connection and twist them in cautiously without force.
—Fill two third of the 500 mL beaker with water.
—Set the delivery glass tube such that the produced gas flow through the water as seen in the Figure.
—Close the stopcock of the separatory funnel and fill it with 3M hydrochloric acid about half full.
Caution: Handle the acid solutions with great care, because they are corrosive and harmful.
—Open the stopcock of the separatory funnel slowly and allow hydrochloric acid to drop onto the sodium car-
bonate until all of the sodium carbonate reacts with the acid.
—Close the stopcock of the separatory funnel and clean the erlenmeyer flask.
Note: Do not discard the water in the beaker.
Note: The above procedure is done to make the water be saturated with carbon dioxide gas. This increases the sensitivity of the experiment.
— Fill the gas measuring tube (or 50 mL graduated cylinder) with water. Close the mouth of the tube with your thumb (or a piece of paper), then sink it into the water in the beaker. Open the mouth of the tube.
—Weigh about 0.2 g of anhydrous sodium carbonate, and record the measurement in the Table in the “Observations and Data Tables”. Then put the sodium carbonate into the erlenmeyer flask.
—Re-assemble the apparatus as seen in the Figure.
Note: Be sure that the delivery tube is set such that the produced gas flows in to the gas measuring tube.
— Wear protective glasses.
Procedure:
1.Close the stopcock of the separatory funnel and fill it with 3M hydrochloric acid about half full.
Experiment – 32 How can molar volume of a gas be determined? |
83 |
|
2.Open the stopcock of the separatory funnel slowly and allow several drops of hydrochloric acid to drop onto the sodium carbonate until all of the sodium carbonate
Note: Be careful to add hydrochloric acid dropwise, otherwise the reaction is too violent.
3.By holding the tube vertically, measure the volume of the gases (carbon dioxide and water vapour) in the gas measuring tube. Record your measurement in Table in “Observations and Data Tables”.
Figure
4.If the level of the water inside of the tube and the level of the water outside of the tube are not equal, read the the difference between the water levels on the gas measuring tube. Record your measurement in the Table in “Observations and Data Tables”.
5.Read the room temperature on the thermometer and record it in the Table in “Observations and Data Tables”.
6.Ask your instructor for the room pressure, then record it in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES
1.Note your observations in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with the your measurements?
Mass of the sodium |
|
Volume of the gas in |
|
Difference between |
|
|
|
|
carbonate |
|
the gas measuring |
|
the water levels |
|
Room temperature |
|
Room pressure |
(m(Na2CO3)) |
|
tube (VExp) |
|
(hWater) |
|
|
|
|
|
|
|
|
|
|
|
|
|
........................ g |
|
........................ mL |
|
........................ cm |
|
........................ °C |
|
........................ atm |
|
|
|
|
|
|
|
|
|
CALCULATIONS
1.Calculate the pressure of the gas mixture (CO2 and water vapour) in the gas measuring tube as below.
If the level of inside water is higher than that of outside : |
PMixture = PRoom |
- |
PWater = |
............ - ............ |
= ............ |
atm |
If the level of inside water is lower than that of outside : |
PMixture = PRoom |
+ |
PWater = |
............ - ............ |
= ............ |
atm |
PWater = (dWater x hWater ) / |
(dMercury x 76) = ( 1 x ............ |
) / ( 13.6 x 76 ) =............. |
atm |
|||
Experiment – 32 How can molar volume of a gas be determined?
84

EXPERIMENTAL SET
2.Carbon dioxide gas was collected in the gas measuring tube with water vapour at that temperature. Therefore the partial pressure of the carbon dioxide is calculated as below.
PMixture |
= PCO |
2 |
+ PWater , then PCO |
= PMixture - PWater vapour = ............ |
- ............ |
= ............ |
atm |
|
|
|
2 |
|
|
|
The vapour pressures of water at different temperatures are given in the Table below.
Temperature |
15 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
28 |
29 |
30 |
40 |
50 |
80 |
90 |
100 |
|
(°C) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pressure |
12.8 |
17.5 |
18.7 |
19.8 |
21.1 |
22.4 |
23.8 |
25.2 |
26.7 |
28.3 |
31.8 |
55.3 |
92.5 |
355.1 |
525.8 |
760.0 |
|
(mmHg) |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.Calculate the mol number of the produced carbon dioxide gas by using balanced reaction equation.
Na2CO3(s) + HCl(aq) |
|
2NaCl(aq) |
|
+ H2O(l) |
+ CO2(g) |
|
|
|
|
|
||
|
|
|
|
|
|
|
||||||
According to reaction equation : n(Na |
CO |
) |
= n(CO ) = m(Na |
CO |
) |
/ MW(Na CO ) = ............ |
/ 106 =............ |
mol |
||||
|
2 |
3 |
|
2 |
2 |
3 |
|
2 |
3 |
|
|
|
4.Find the molar volume of produced CO2 gas (V2) at room temperature by using the Avogadro’s principle;
V(CO |
) |
|
n(CO |
) |
|
|
|
|
|
|
2 |
|
|
2 |
|
(Where; V2 |
= Molar volume of produced CO2 at room temperature and n2 = 1 mol) |
||||
———— = |
———— |
|||||||||
V2 |
|
|
n2 |
|
|
|
|
|
|
|
So V2 = (V(CO ) |
x n2) / n(CO |
) = ( ............ |
x 1 ) / ............ |
= ............ |
mL = ............ |
L |
||||
|
|
2 |
|
|
2 |
|
|
|
|
|
5.Calculate the molar volume of carbondioxide at standard conditions.
V(STP) |
P(CO2) x V(CO2) x T(STP) .............. |
x ............. |
x 273 |
L |
|
|||
= ——————————— = ——————————————— = |
|
|||||||
|
|
P(STP) x T(Room) |
1 x ............. |
|
|
|
|
|
6. Calculate the error in the experiment. |
|
|
|
|
|
|
||
% Error = |
|True value – Experimental value| |
|
x 100 = |
|22.4 – ............... |
| |
x 100 = |
% |
|
True value |
|
|
|
|||||
|
|
|
|
..................... |
|
|
|
|
EVALUATIONS AND CONCLUSIONS 


1.If the error is high, explain the reasons.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Compare your result with that of the other groups and give reasons for the differences.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Why does the equal mol number of any gas has the same volume at the same conditions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 32 How can molar volume of a gas be determined? |
85 |
|
How fast gas molecules move? (Graham’s law)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the relation between velocity of gas particles and molecular mass.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other materials |
• Glass tube, 10 mm x 80 cm |
(1) |
• Support base |
(1) |
• Hydrochloric acid, concentrated |
• Watch glasses, |
(2) |
• Universal Clamp |
(1) |
• Ammonia, concentrated |
• Dropper |
(1) |
• Bosshead |
(1) |
• Cotton |
• Support rod |
(1) |
• Rubber stopper |
(2) |
|
PRE-LAB DISCUSSION
Diffusion is the complete spreading out and intermingling of the molecules of a gas with another. Perfume or cologne diffuses in the air in a room. We can think of this process as the movement of perfume molecules from a region of higher partial pressure to lower.
A similar process called effusion, is the movement of gas molecules through an extremely tiny hole into a region of lower pressure. Thomas Graham (1805-1869) a scottish scientist studied the rates of effusion of a series of gases through the same tiny hole, and he found that the more dense a gas is, the slower it effuses. The relationship is called Graham’s Law.
The molecules of gases are in rapid motion and the distance between molecules is very great. Therefore, a gas can mix with or move through another gas. Graham found that the rates of diffusion of gases vary inversely with the square roots of their molecular mass.
In this experiment you will allow ammonia and hydrogen chloride gases to diffuse through air in a glass tube. The formation of white deposit on the wall of the tube will indicate the point where the two gases meet. The diffusion rate of the two gases is the comparison of the distances they travel per unit time through the glass tube.
PROCEDURE
Set-up
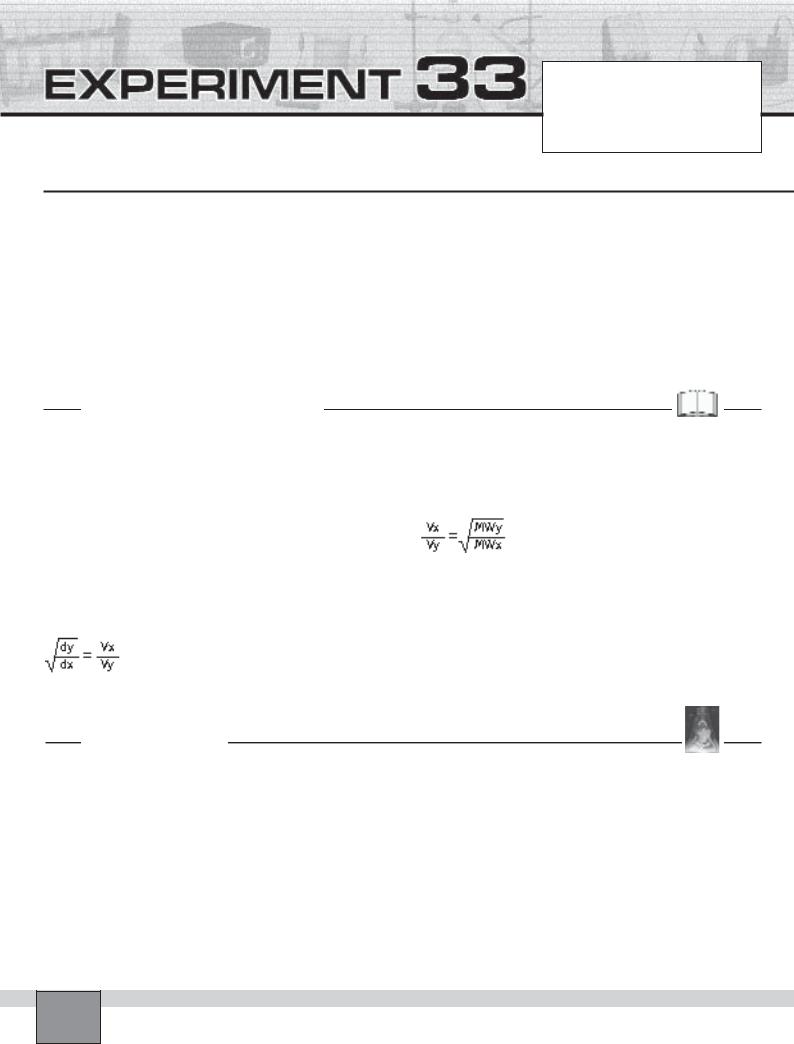
—Obtain a 80 cm glass tube. Make sure that, it is completely dry. Clamp the tube as seen in the Figure on the support rod.
—Fix some cotton onto the each rubber stopper with pins so that they fit the opening ends of the tube. Place the cotton swabs in each end of the tube.
—Mark the glass tube to indicate the position of the end of each swab as illustrated in the Figure.
—Take the cotton swabs out and put them on different watch glasses.
Procedure:
1.By using dropper, add about five drops of concentrated hydrogen chloride on one of the cotton swabs and five drops of concentrated ammonia on the other swap.
Caution:Handle always acids and bases with great care. Do not attempt to inhale the vapours.
2.Immediately and simultaneously insert the moistened end of the cotton swabs into the openings of the tube, to the line previously marked.
3.Observe the formation of white ring in the glass tube. Record your observations in “Observations and Data Tables”.
Experiment – 33 How fast gas molecules move? (Graham’s law)
86
4.Mark the point on the tube where the white ring is formed.
5.Measure the distance travelled by each gas by the ruler and record your measurements in the Table in “Observations and Data Tables”.
Figure
6. Repeat the Experiment if the time permits.
Note: Since hydrochloric acid and ammonia produce unpleasant smelling and harmful vapours, Air the room after experiment.
OBSERVATIONS AND DATA TABLES
1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with your measurements?
|
|
|
|
|
1. Experiment |
|
2. Experiment |
|
Average |
|
|
|
|
|
|
|
|
|
|
Distance travelled by HCl |
(XHCl) |
|
|
.......................... cm |
|
.......................... cm |
|
.......................... cm |
|
|
|
|
|
|
|
|
|
|
|
Distance travelled by NH3 |
(X |
NH3 |
) |
|
cm |
|
cm |
|
cm |
|
|
|
|
|
|
||||
CALCULATIONS
1.Calculate the ratio of the diffusion rates of NH3 and HCl gases.
Experiment – 33 How fast gas molecules move? (Graham’s law) |
87 |
|

2.Calculate the experimental ratio of molecular weights of NH3 and HCl by using Graham’s law.
EVALUATIONS AND CONCLUSIONS 


1.Was Graham’s law verified by this experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Compare the experimental ratio of molecular masses with theoretical ratio.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Based on the experiment, compare and explain?
a)Velocities of gases :.........................................................................................................................................................
...........................................................................................................................................................................................
b)Molecular weights of gases :...........................................................................................................................................
...........................................................................................................................................................................................
c)Densities of gases at the same conditions :....................................................................................................................
...........................................................................................................................................................................................
4.What is the compound that forms in the tube? Write the balanced reaction equation.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 33 How fast gas molecules move? (Graham’s law)
88
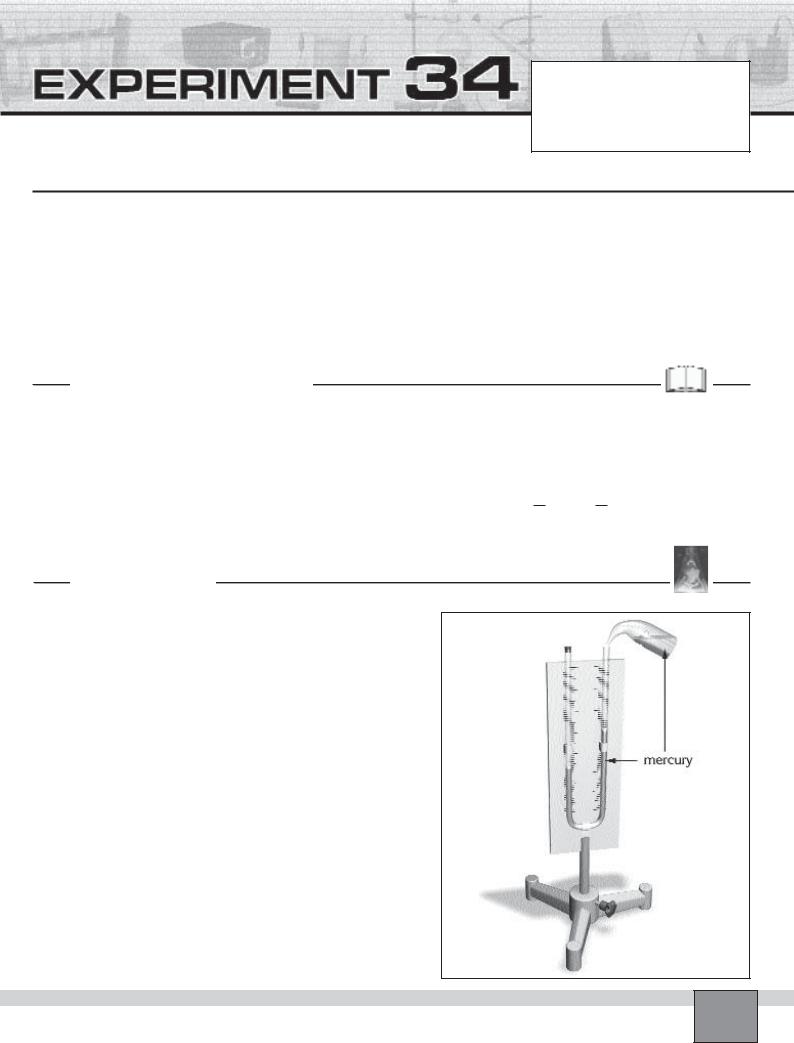
How does pressure affect the volume of a gas? (Boyle’s Law)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the relation between pressure and volume of a gas.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Thermometer |
(1) |
• Barometer |
(1) |
• Mercury |
• Mercury dropper |
(1) |
• Support base |
(1) |
|
• Manometer |
(1) |
• Rubber stopper, without hole |
(1) |
|
PRE-LAB DISCUSSION
Scientists showed that gas pressure is the net effect of innumerable collisions made by gas particles on the walls. Suppose that one wall of a gas container is a movable piston that can be pushed in (or pulled out). If the volume is decreased without changing the temperature, no change would occurs in the average kinetic energy of the molecules. But there would be more gas particles per unit volume. If the volume
is reduced by one half the number of molecules per volume will double. This change, doubles the number of collisions per second with a unit area. Therefore the pressure is doubled, which is exactly what Boyle discovered. The relation between pressure and volume is represented as below
P 1V or V P1
PROCEDURE
Figure
Set-up
—Clamp the manometer onto the support rod.
—Fill one fourth of the manometer with mercury by mercury dropper.
Caution: Do not swallow and touch mercury or inhale the mercury vapour because it is extremely harmful.
Note: Do this experiment under the hood.
—Close the opening of the left column of the manometer with a stopper as seen in the Figure.
Procedure
1.Measure the room temperature and pressure, and record them in the Table in “Observations and Data Tables”.
2.Read the height of the trapped air in the manometer and the difference between mercury columns. Record your measurements in the Table in “Observations and Data Tables”.
Experiment – 34 How does pressure affect the volume of a gas? (Boyle’s Law) |
89 |
|

3. Add some mercury to the right column with mercury |
4. Repeat step 3 three more times and record your mea- |
|
dropper. Then read the height of the trapped air and |
surements in the Table in “Observations and Data Ta- |
|
the difference between mercury columns. Record your |
bles”. |
|
measurements in Table in “Observations and Data Ta- |
Caution: Air the room after the experiment. |
|
bles”. |
||
|
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with your measurements.
Measurements |
|
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Height of trapped air (hair) |
|
cm |
|
cm |
|
cm |
|
cm |
|
cm |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Mercury level difference (hHg) |
|
cm |
|
cm |
|
cm |
|
cm |
|
cm |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Room Temperature |
|
|
|
oC |
|
|
|
|
|
|
|
|
.............. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Room Pressure (PRoom) |
|
|
|
cmHg |
|
|
|
|
|
|
|
|
.............. |
|
|
|
|
|
|
|
CALCULATIONS
1.Calculate the pressure of trapped air for each measurement by using the formula below and fill the Table.
PTrapped air = PRoom + hHg |
|
|
|
|
|
|
|
|
|
|
Measurements |
|
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
|
|
|
|
|
|
|
|
|
|
Pressure of trapped air (PTrapped air) |
|
.......... cmHg |
|
.......... cmHg |
|
.......... cmHg |
|
.......... cmHg |
|
.......... cmHg |
2.Plot the graph of pressure against volume by using experimental values ( Since height and volume are directly proportional, height of the trapped air can be used as volume) on the graph paper next.
3.Select three points on the graph and calculate three P x V values and write in the Table.
No |
|
Pressure |
|
Volume |
|
P x V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
.......... cmHg |
|
.......... cm3 |
|
.............. |
|
|
|
|
|
|
|
2 |
|
.......... cmHg |
|
.......... cm3 |
|
.............. |
|
|
|
|
|
|
|
3 |
|
.......... cmHg |
|
.......... cm3 |
|
.............. |
Experiment – 34 How does pressure affect the volume of a gas? (Boyle’s Law)
90

EVALUATIONS AND CONCLUSIONS 


1.Was Boyle’s law verified by this experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How can you perform the experiment if you use water instead of mercury? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Why the temperature of the trapped air was kept constant? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 34 How does pressure affect the volume of a gas? (Boyle’s Law) |
91 |
|

How does temperature affect the volume of a gas? (Charles’s Law)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the relation between temperature and volume of a gas.
EQUIPMENT and MATERIALS:
Equipment |
|
• Universal clamp |
(1) |
• Beaker, 250 mL |
(1) |
• Bosshead |
(1) |
• Beaker, 100 mL |
(1) |
• Wire gauze |
(1) |
• Thermometer |
(1) |
• Tripod |
(1) |
• Mercury dropper |
(1) |
• Burner |
(1) |
• Glass tube, with one opening, 30 cm |
(1) |
• Test tube holder |
(1) |
• Barometer |
(1) |
Chemicals and Other Materials |
|
• Support rod |
(1) |
• Mercury |
|
• Support base |
(1) |
• Ruler |
|
PRE-LAB DISCUSSION
Temperature - Volume law (Charle’s law) :
The kinetic theory tells us that increasing the temperature of a gas increases the kinetic energy of its particles which causes increase in the pressure of the gas. The only way to keep the pressure constant is to allow the gas to expand.
Therefore, a gas expands with increasing temperature in order to keep pressure constant. In an other word, V T at constant pressure. This is how the kinetic theory explains Charle’s law.
PROCEDURE
Set-up |
Figure |
—Fill two third of the 250 mL beaker with water, then put it on the wire gauze on the tripod.
—Place some amount of mercury in a 100 mL beaker.
Caution: Do not swallow and touch mercury or inhale the mercury vapour because it is extremely harmful.
Note: Do this experiment under the hood.
—Hold the glass tube with a holder and heat the closed end of the glass tube over heater gently to make the air go out.
—Stop heating and turn the opening end of the tube down, then sink the opening of the tube into the mercury in the beaker so that it absorbs a bit of mercury inside.
—Turn the closed end of the tube down to trap some air in the tube.
—Make height of the trapped air about 1 cm.
Note: Tilt the glass tube slowly and allow the mercury goes toward the bottom of the tube. Make the tube vertical if the height of the trapped air is about 1 cm.
Experiment – 35 How does temperature affect the volume of a gas? (Charles’s Law)
92
