
experment book
.pdf
Do solutions conduct electricity?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe that atom contains positive and negative charged particles.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 250 mL |
(4) |
• Potassium chloride solution |
• 6 V bulb |
(1) |
• Copper sulphate solution |
• Plug-in bulb mouth |
(1) |
• Sugar solution |
• Steel electrode |
(2) |
• Distilled water |
• Crocodile leads |
(3) |
• Paper tissues or cloth |
• Power supply, (0-12 V, DC) (1)
PRE-LAB DISCUSSION
Electric current is a flow of electrons between two poles having different electric potential. The electron flow is carried by free electrons of metals if they are used as wire. On the other hand, electrons are carried by ions between poles in a so-
lution. Therefore, if a solution of a substance conducts electricity, it means that, the substance is decomposed into ions. Ions are positively or negatively charged particles. That is, substances consist of positive and negative particles.
PROCEDURE
Set-up
—Place distilled water, potassium chloride solution, copper chloride solution and sugar solution into four different 250 mL beaker. Then label them.
—Arrange the circuit as shown in the Figure for distilled water.
Figure
Caution: Do not switch the power supply on before setting up the apparatus completely.
Note: Be careful that the electrodes do not contact each other in the solution.
Experiment – 20 Do solutions conduct electricity? |
53 |
|
Procedure
1.Switch the power supply on to apply 6 V. Observe the bulb that if it is lightening or not. Record your observation in the Table in “Observations and Data Tables”.
2.Switch the power supply off and take the electrodes out. Examine the electrodes. Record your observation
in the Table in “Observations and Data Tables”. Dry the electrodes with paper tissues.
3.Place the electrodes in potassium chloride solution and repeat the steps 1 and 2.
4.Repeat the steps 1 and 2 for the the other two solutions.
OBSERVATIONS AND DATA TABLES 


1.Note your observations on the conductivity of the solutions below.
Distilled water
KCl solution
Bulb (on/off)
2.Note your observations on the electrodes after conductivity test.
Distilled water
KCl solution
Appearance of electrodes
CuSO4 |
|
Sugar |
solution |
|
solution |
|
|
|
|
|
|
CuSO4 |
|
Sugar |
solution |
|
solution |
|
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS 


1.How does the salt solution conduct electricity? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What can be the reason that the sugar solution do not conduct electricity?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What kind of water solutions of compounds conduct electricity? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.How does the the electrical conductivity of the salt solution support that an atom includes charged particles?.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 20 Do solutions conduct electricity?
54
Is water attracted by electrostatically charged objects?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To show and prove that water molecules have positive and negative poles.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Beaker, 500 mL |
(1) |
• Support rod |
(1) |
• Water |
• Buret |
(1) |
• Buret clamp |
(1) |
• Ebonite rod (or nylon comb) |
• Support base |
(1) |
• Bass head |
(1) |
|
PRE-LAB DISCUSSION
Water molecules consist of two hydrogen atoms and one oxygen atom. Hydrogen atoms in water molecule are positively charged and oxygen atom in a water molecule is negatively charged. Since the shape of the water molecule is,
water molecules are polar molecules which have positive and negative poles.
|
PROCEDURE |
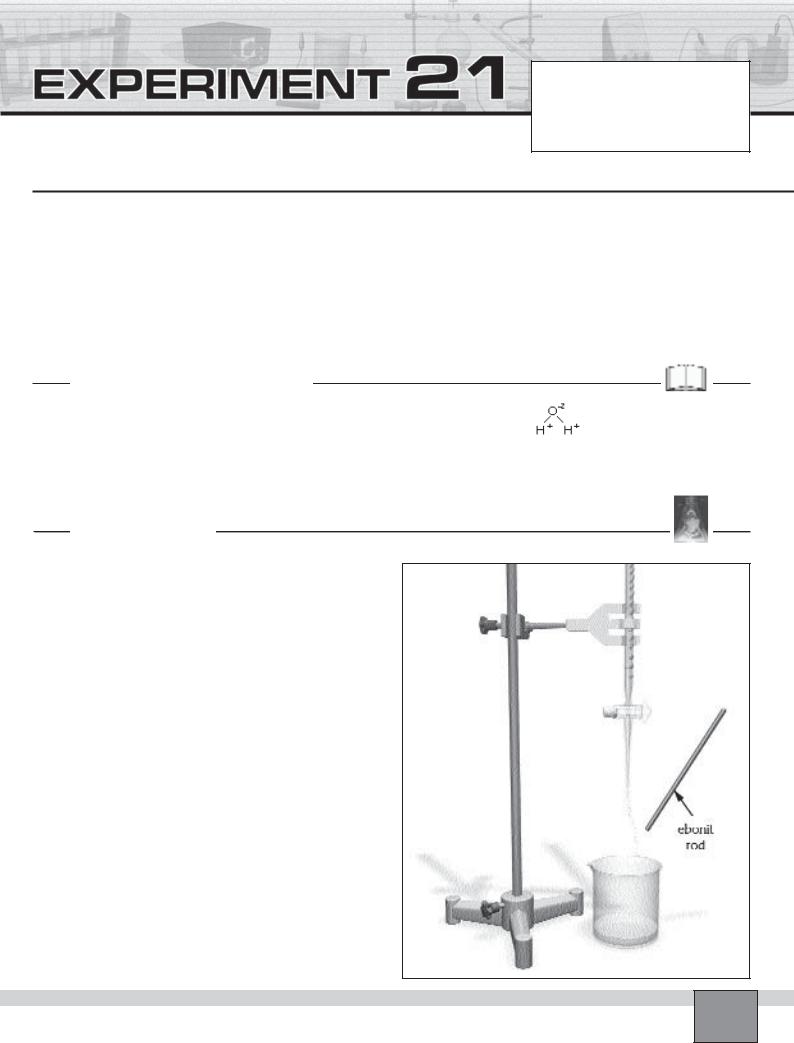
Set-up |
Figure |
—Set up the apparatus as seen in the Figure.
—Fill the buret with tap water.
Procedure
1.Arrange for a thin, steady stream of water to run from the buret into the beaker.
2.Rub briskly the ebonite rod against the hair, or any electrostatic demonstration materials.
3.Hold the electrostatically charged ebonite rod near the water stream.
4.Observe what happens to the water stream. Record your observations in “Observations and Data Tables”.
Experiment – 21 Is water attracted by electrostatically charged objects? |
55 |
|

OBSERVATIONS AND DATA TABLES 


1.Note your observations, What happens to the water stream when ebonite rod is approached to it?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.What is the possible reason for bending of water stream?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Would all liquids behave in a similar manner? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What conclusion can you draw from your observations?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 21 Is water attracted by electrostatically charged objects?
56
What happens when metals are heated with sulphur?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To investigate the differences between elements and its compounds.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(2) |
• Support rod |
(1) |
• Copper foil |
• Watch glass |
(1) |
• Universal clamp |
(1) |
• Iron filings |
• Test tube rack |
(1) |
• Bosshead |
(1) |
• Sulphur |
• Burner |
(1) |
• Protective glasses |
(1) |
• Scissors |
• Support base |
(1) |
|
|
• Tweezers |
|
|
|
|
• Sheet of paper |
PRE-LAB DISCUSSION
Chemical reactions are different from physical processes. In chemical reactions, substances are subject to permanent changes; new substances with new properties are formed.
This can be seen in the change of colour, density and other properties.
PROCEDURE
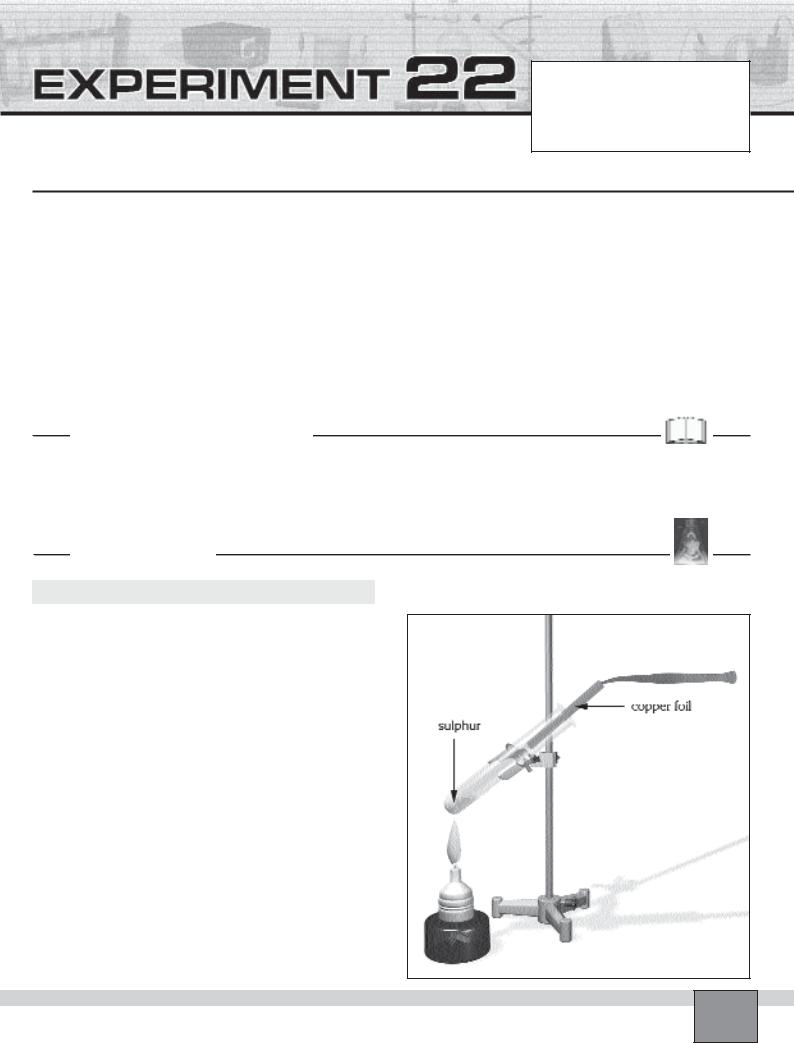
Part-A : Does Copper React with Sulphur
Figure
Caution: No experiments other than Fe and Cu should be attempted. Because the reactions between sulphur and some elements (e.g. magnesium) are extremely vigorous. They should not be done even as a demonstration. (Zinc powder can be used for demonstration.)
Set-up
—Place a test tube in the test tube rack. Fill the test tube about 1 cm high with a small pieces of sulphur.
—Cut a strip of bright thin copper foil with scissors.The strip should be 2 cm wide and 5 cm long.
—Bend the strip longitudinally to form a channel that suitable for the test tube.
—Wear protective glasses.
Procedure
1.Examine the copper foil and sulphur, then note your observations in the Table in “Observations and Data Tables”.
2.Heat the test tube in order to boil sulphur and form vapour of sulphur which does not condense immediately.
Experiment – 22 What happens when metals are heated with sulphur? |
57 |
|

Note: Heat the test tube from the bottom up to two-thirds of its height. Fist heat the sulphur, then heat the middle of the test tube.
Caution: No sulphur should escape from the test tube. It may ignite in air.
3.Wait until the test tube is about half-filled with sulphur vapour.
Note: Ensure that an adequate amount of sulphur vapour is present in test tube. Otherwise the spontaneous reaction does not take place.
4.Hold the copper foil with a tweezers and insert it into the test tube. Let the foil comes in contact with the sulphur vapour.
5.Allow the test tube to cool and observe the copper foil strip. Record your observations in Table in “Observations and Data Tables”.
Note: Since sulphur produces unpleasant smelling and harmful vapours on heating, Air the room after experiment.
Part-B : Does Iron React with Sulphur
Set-up
—Weigh 2.1 g of iron filings and 1.2 g of sulphur. Place them on different piece of papers and observe the appearance of elements.
—Hold a magnet over the elements. Record your observations in the Table “Observations and Data Tables”.
—Mix them on a watch glass. Place the mixture in a test tube.
—Wear protective glasses
Procedure
1.Heat the mixture by placing the burner underneath.
Figure-1
2.Withdraw the test tube as soon as red glow begins to spread through the mixture. The reaction will complete itself without further heating.
3.Place the product on the watch glass. Examine the appearance and magnetic property of it. Record your observations in the Table “Observations and Data Tables”.
Note: Some sample of iron filings may be found not to react with sulphur under the experiment conditions.
Since sulphur produces unpleasant smelling and harmful vapours on heating, Air the room after experiment.
Figure-2
Experiment – 22 What happens when metals are heated with sulphur?
58
OBSERVATIONS AND DATA TABLES 


Part-A
1.Note your observations about copper and sulphur reaction.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below (Use melting point and density tables).
Substance |
|
Colour |
|
Density (g/cm3) |
|
Melting point (° C) |
|
|
|
|
|
|
|
Copper |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sulphur |
|
|
|
|
|
|
|
|
|
|
|
|
|
Copper(II) sulphide |
|
4.6 |
|
|
|
Decompose before melting |
|
|
|
|
|
|
|
Part-B
1.Note your observations about iron and sulphur reaction.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below (Use melting point and density tables).
Substance |
|
Colour |
|
Effect of magnet |
|
Density (g/cm3) |
|
Melting point (oC) |
|
|
|
|
|
|
|
|
|
Iron |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sulphur |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron(II) sulphide |
|
|
|
|
|
4.84 |
|
1466 |
|
|
|
|
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS
1.Compare the properties of the newly produced substance with its starting elements in Part-A
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Compare the properties of the newly produced substance with its starting elements in Part-B
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.How can chemical changes be recognised? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What are differences between chemical and physical changes? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 22 What happens when metals are heated with sulphur? |
59 |
|

Law of definite proportions
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the mass ratio of magnesium and oxygen in magnesium oxide.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Crucible, with cover |
(1) |
• Burner |
(1) |
• Magnesium ribbon, 15-30 cm |
• Clay triangle |
(1) |
• Balance |
(1) |
• Ruler |
• Tripod |
(1) |
• Crucible, tongs |
(1) |
|
PRE-LAB DISCUSSION
There is a definite proportion between the atoms that form a compound. For example; water is formed from hydrogen and oxygen and always contain 2 g of hydrogen for every 16 g of oxygen. Thus the ratio of hydrogen to oxygen by mass is 1/8. Similarly, oxygen to copper in CuO by mass is 1/4 and the ratio of sulphur to iron in FeS by mass is 4/7.
French chemist Proust stated that “There is a constant ratio between the elements in a compound.”
From this ratio we can deduce that formula and compounds of a composition are always definite. For example, burning of carbon (C) with oxygen of air produces carbon dioxide (CO2), and burning of petroleum with oxygen of air pro-
duces carbon dioxide (CO2) too. Those carbon dioxides produced by two different reactions have the same molar mass, and formula (CO2).
PROCEDURE
Set-up
—Cut 5 cm long and 10 cm long of magnesium ribbon. Make them into a tight coil.
Note: Scrape the surface of the ribbon so that it is free from oxide.
—Weigh clean and dry crucible with its cover. Record the value in the Table in “Observations and Data Tables”.
—Take 5 cm magnesium ribbon and place it in the crucible, then weigh the whole, not forget the cover. Record the value in the Table in “Observations and Data Tables”.
—Set the apparatus as seen in the Figure.
—Wear protective glasses.
Procedure
1.Heat the crucible by means of the burner until the magnesium ignites.
Note: The flame should be low at the beginning, then should be raised gradually until the magnesium ignites.
The flame should never be too big. The loss of magnesium oxide will be excessive.
2.Remove the burner and raise the crucible cover for a few moments at a time with tongs so that air may be admitted.
Note: Take care that as little smoke as possible escapes from crucible.
Figure
Experiment – 23 Law of definite proportions
60

3.Repeat the heating, raising and lowering of the cover in order to be sure that all the magnesium has burned.
4.As soon as the the magnesium stoped to flare up and appears to have finished burning, remove the cover and heat the crucible strongly to make be sure that the combustion is complete.
5.Stop heating and allow the crucible to cool. Then re-
weigh the crucible with its content and cover. Record the value in the Table in “Observations and Data Tables”.
6.Repeat step-1 to step-5 for the 10 cm long of magnesium ribbon. Record the values in the Table in “Observations and Data Tables”.
Note: The more amount of magnesium ribbon qives the more sensitive result in this experiment.
OBSERVATIONS AND DATA TABLES 


1.Note your observations
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write your measurements in the Table below.
|
|
For 5 cm long Mg ribbon |
|
For 10 cm long Mg ribbon |
|
|
|
|
|
|
|
|
|
|
Wt of Crucible and cover (W1) |
|
..................................... g |
|
..................................... g |
|
|
|
|
|
Wt of Crucible, cover and magnesium (W2) |
|
..................................... g |
|
..................................... g |
|
|
|
|
|
Wt of Crucible, cover and magnesium oxide (W3) |
|
..................................... g |
|
..................................... g |
EVALUATIONS AND CONCLUSIONS
1.Calculate the values and fill the Table below by using the data in the Table in “Observations and Data Tables”.
|
|
|
|
For 5 cm long Mg ribbon |
|
|
For 10 cm long Mg ribbon |
|
|
|
|
|
|
|
|
Mass of Magnesium (mMg) (W2 - W1 ) |
|
.................... g |
|
|
.................... g |
||
|
|
|
|
|
|
|
|
Mass of Oxygen (mO) (W3 - W2 ) |
|
|
|
.................... g |
|
|
.................... g |
|
|
|
|
|
|
|
|
mMg / mO ratio |
|
|
|
.................. / ................... |
|
|
.................. / ................... |
|
|
|
|
|
|
|
|
Average mMg / mO ratio |
|
(Ratio(5 cm Mg) + Ratio(10 cm Mg)) / 2 = ( ............. |
+ |
............. ) / 2 = ............ |
|||
2.Compare the your mMg / mO ratios with each other and with friend’s results. If they are not similar, what reasons can make them different? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What can you deduce from the mMg / mO ratio? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Compare experimental average mMg / mO ratio with the theoretical value. Calculate the percent error? What may cause the differences?
...........................................................................................................................................................................................
%Error=( Theoretical value - Exp. average value Theoretical value) x 100 = ( ........ |
- ........ |
/ |
........) x 100 = |
.......% |
Experiment – 23 Law of definite proportions |
61 |
|

What is lost when copper(II) sulphate crystals are heated?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : |
To find out what is lost when copper(II) sulphate crystals are heated. |
|
||||
|
To find out mass ratio of water to copper(II) sulphate in hydrated copper(II) sulphate salt. |
|
||||
EQUIPMENT and MATERIALS: |
|
|
|
|||
|
|
|
|
|
|
|
Part-A |
|
|
|
|
|
|
Equipment |
|
|
• Rubber tubing, 10 cm |
(1) |
• Mortar with pestle |
(1) |
• Beaker, 500 mL |
|
(1) |
• Rubber stopper, with one hole (1) |
• Spatula |
(1) |
|
• Test tube |
|
(2) |
• Burner |
(1) |
• Protective glasses |
(1) |
• Thermometer |
|
(1) |
• Support base |
(2) |
Chemicals and Other Materials |
|
• Watch glass |
|
(1) |
• Support rod |
(2) |
• Copper(II) sulphate, hydrated |
|
• Glass tube |
|
(1) |
• Universal clamp |
(2) |
• Cold water |
|
• Right - angled glass |
(1) |
• Bosshead |
(2) |
|
|
|
|
|
|
|
|
|
|
Part-B |
|
|
|
|
|
|
Equipment |
|
|
|
|
Chemicals and Other Materials |
|
• Evaporating dish |
|
(1) |
• Spatula |
(1) |
• Copper(II) sulphate, hydrated |
|
• Tripod |
|
(1) |
• Mortar with pestle |
(1) |
|
|
• Balance |
|
(1) |
|
|
|
|
PRE-LAB DISCUSSION
The strong dipole of water causes water molecules to attach |
Hydrated compounds are called hydrates in general. The |
themselves to ions. Such ions are called hydrated ions. When |
number of moles of water present per mole of anhydrous salt |
the hydrated ions are heated, the water molecules evaporate |
is usually simple number. |
and crystallisation of ion occurs. The crystals change form, |
|
sometimes, even colour, as the water driven off. |
|
|
|
PROCEDURE
Part-A
Set-up
—Finely powder the salt of copper(II) sulphate crystals in the mortar. Fill one of the test tubes about half full with powdered copper(II) sulphate.
—Make the connection of rubber stopper and delivery glass tube.
Note: Lubricate glass-rubber connections with glycerol before connection.
—Fill two third of the 500 mL beaker with cold water.
—Set up the apparatus as seen the Figure.
Procedure
1.Heat the test tube gently until the liquid collects in the test tube in the cold water. Note your observations in the “Observations and Data Tables”.
Note: About 2 cm depth of liquid is expected from this amount of salt.
Caution: Be careful to keep the end of the delivery tube above the collected liquid. Otherwise, some of the liquid may draw back into the heated tube.
2.Continue heating until no more liquid vapour is driven off.
Experiment – 24 What is lost when copper(II) sulphate crystals are heated?
62
