
experment book
.pdf
OBSERVATIONS AND DATA TABLES 


Part-A
1. Note the colour of the solutions. |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Ions |
|
|
Fe+3 |
|
SCN– |
|
FeSCN+2 |
|
|
|
|
|
|
|
|
|
|
Colour of Ion |
|
|
|
|
.......................................... |
|
.......................................... |
|
|
|
.......................................... |
|
|
|
||
2.Fill the table below with the comparison of the colours of the solutions with reference solution in test tube 1.
Added solutions |
|
KSCN in test tube 2 |
|
FeCl3in test tube 3 |
|
KCl in test tube 4 |
|
NaOH in test tube 5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in colour |
|
|
|
|
|
|
|
|
(lighter or darker) |
|
................................ |
|
................................ |
|
................................ |
|
................................ |
|
|
|
|
|
|
|
|
|
Part-B
1.Note the colour of the solutions.
Ions |
|
CrO4-2 |
|
Cr2O7-2 |
|
|
|
|
|
Colour of Ion |
|
|
|
......................... |
|
|
......................... |
|
2.Fill the table below with the colour of the solutions.
|
|
|
First addition |
|
Second addition |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Added solutions |
|
HCl |
|
|
NaOH |
|
HCl |
|
NaOH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Colour of the solution |
|
|
|
|
|
|
|
|
|
(Chromate or dichromate) |
|
......................... |
|
|
..................... |
|
..................... |
|
..................... |
EVALUATIONS AND CONCLUSIONS
Part-A
1.Write the chemical equilibrium equation for the reversible reactions that took place in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Using Le Chatelier’s principle, explain how did the addition of solutions affect the equilibrium. In which direction was the equilibrium shifted?
Addition of |
KSCN |
: ......................................................................................................................................................... |
Addition of |
FeCl3 |
: ......................................................................................................................................................... |
Addition of |
KCl |
: ......................................................................................................................................................... |
Addition of |
NaOH |
: ......................................................................................................................................................... |
Experiment – 50 How does the addition of a substance disturb an equilibrium reaction? |
133 |
|

3.Did the concentration of the Fe+3, SCN- and FeSCN+2 ions increase or decrease?
|
|
Fe+3 |
SCN- |
FeSCN+2 |
|
|
_____________________ |
_____________________ |
_____________________ |
After addition of |
KSCN |
: ........................... |
........................... |
........................... |
After addition of |
FeCl3 |
: ........................... |
........................... |
........................... |
After addition of |
KCl |
: ........................... |
........................... |
........................... |
After addition of |
NaOH |
: ........................... |
........................... |
........................... |
4.Does the addition of any salt in the equilibrium reaction disturb the equilibrium? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Part-B
1.Is the reaction an equilibrium reaction? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.In which direction was the equilibrium shifted?
After addition of |
HCl |
: ............................................................................................................................. |
After addition of |
NaOH |
: ............................................................................................................................. |
3.How did the concentration of the CrO4-2 and Cr2O7-2 ions change? (Increased or decreased)
|
|
CrO -2 |
Cr |
O -2 |
|
|
4 |
2 |
7 |
After addition of |
HCl |
: ........................... |
........................... |
|
After addition of |
NaOH |
: ........................... |
........................... |
|
4.Does the experiment prove the Le Chatelier’s principle? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Does the disturbance of an equilibrium reaction change the equilibrium constant Kc by addition of substances?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 50 How does the addition of a substance disturb an equilibrium reaction?
134
How does temperature affect an equilibrium reaction?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the effect of temperature on an equilibrium reaction.
EQUIPMENT and MATERIALS:
Equipment |
|
• Wire gauze |
(1) |
Chemicals and Other Materials |
• Test tube |
(1) |
• Burner |
(1) |
• Copper(II) nitrate, 1M |
• Beaker, 250 mL |
(2) |
• Stirring rod |
(1) |
• Sodium chloride salt |
• Tripod |
(1) |
• Spatula |
(1) |
• ice |
PRE-LAB DISCUSSION
All chemical reactions involve either the evolution or the absorption of energy. In every system in equilibrium an exothermic and endothermic reaction occur simultaneously. The endothermic reaction is favoured by an increase in temperature and the exothermic reaction is favoured by a
decrease in temperature. Therefore, when the temperature of a reaction in equilibrium is increased, the equilibrium is displaced in such way that heat is absorbed. This is a special case of Le Chatelier’s principle.
PROCEDURE
Set-up
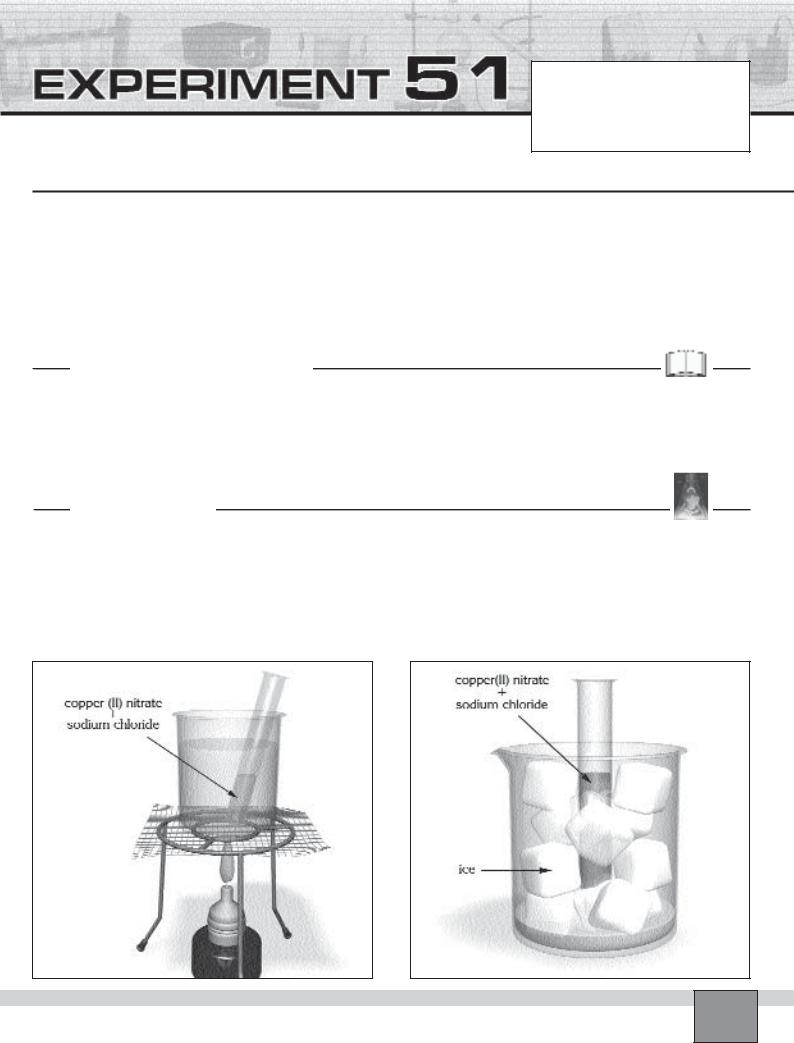
—Place a test tube in the rack and add 3 cm depth of 1M copper(II) nitrate solution in a test tube.
—Add a half spatula of sodium chloride salt in the solution and stir well with a stirring rod. Record the colour of the solution in “Observations and Data Tables”.
Figure-1
—Fill the half of 250 mL beaker with ice-water mixture.
—Fill the half of another 250 mL beaker with tap water and place on the wire gauze on the tripod as seen in Figure-1.
Figure-2
Experiment – 51 How does temperature affect an equilibrium reaction? |
135 |
|

Procedure
1.Take the test tube containing solution and place it into the water in the beaker on the tripod.
2.Ignite the burner and heat the solution by heating water in the beaker.
3.When the colour of the solution change, extinguish the burner. Record the colour of the solution in the test tube in “Observations and Data Tables”.
4.Take the test tube out from the hot water and place it into the ice-water mixture in the beaker.
5.Wait for the colour change and record the colour of the solution in the test tube in “Observations and Data Tables”.
6.If time allows, repeat the steps from 1 to 5.
OBSERVATIONS AND DATA TABLES 


1.Note your observations in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with the colour of the solution during experiment.
|
|
Initial |
|
In hot water |
|
In ice bath |
|
|
|
|
|
|
|
Colour |
|
|
|
........................ |
|
........................ |
|
|
........................ |
|
|
EVALUATIONS AND CONCLUSIONS 


1.If Cu(H2O)4+2 is blue and CuCl4-2 is green, write the equilibrium reaction equation.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.In which direction the reaction is exothermic?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Does the increase in temperature disturb always the equilibrium in the same direction? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Does the change in temperature affect the equilibrium constant Kc? Explain How.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 51 How does temperature affect an equilibrium reaction?
136
Can salt solutions react when they are mixed?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To form insoluble salt by mixing salt solutions.
To determine the substance that forms by mixing of two solutions.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
|
• Test tube |
(5) |
Group 1 |
Group 2 |
• Dropper |
(5) |
• Iron(III) chloride, 0.1M |
• Barium nitrate, 0.1M |
• Glass plate, 20x20 cm |
(1) |
• Lead(II) nitrate, 0.1M |
• Sodium sulphate, 0.1M |
• Test tube rack |
(1) |
• Barium nitrate, 0.1M |
• Barium chloride, 0.1M |
• Black paper sheet, 20x20 cm |
(1) |
• Sodium hydroxide, 0.1M |
• Aluminum chloride, 0.1M |
|
|
• Potassium iodide, 0.1M |
• Strontium nitrate, 0.1M |
PRE-LAB DISCUSSION
Solubility of salts in water are different. They may be soluble, and slightly soluble in water. In some chemical reactions, formation of solid occurs in solution. A slightly soluble salt will precipitate if its ions are present in solution in such quantities that their concentrations exceeds the quan-
tity of saturation. Therefore, when two solutions which contain ions of slightly soluble salt, are mixed, precipitation usually occurs only if the concentration of ions in the new mixture is higher than the that of saturation point.
PROCEDURE
Set-up
Group 1
—Put five test tubes in the rack and place the solutions below in individual test tube and number them.
Solution 1 : 0.1 M FeCl3
Solution 2 : 0.1 M Pb(NO3)2
Solution 3 : 0.1 M Ba(NO3)2
Solution 4 : 0.1 M NaOH
Solution 5 : 0.1 M KI
Group 2
—Put five test tubes in the rack and place the solutions below in individual test tube and number them.
Solution 1 : 0.1 M Ba(NO3)2
Solution 2 : 0.1 M Na2SO4
Solution 3 : 0.1 M BaCl2
Solution 4 : 0.1 M AlCl3
Solution 5 : 0.1 M Sr(NO3)2
—Put black paper on the bench and place the glass plate on the paper.
Experiment – 52 Can salt solutions react when they are mixed? |
137 |
|
Procedure
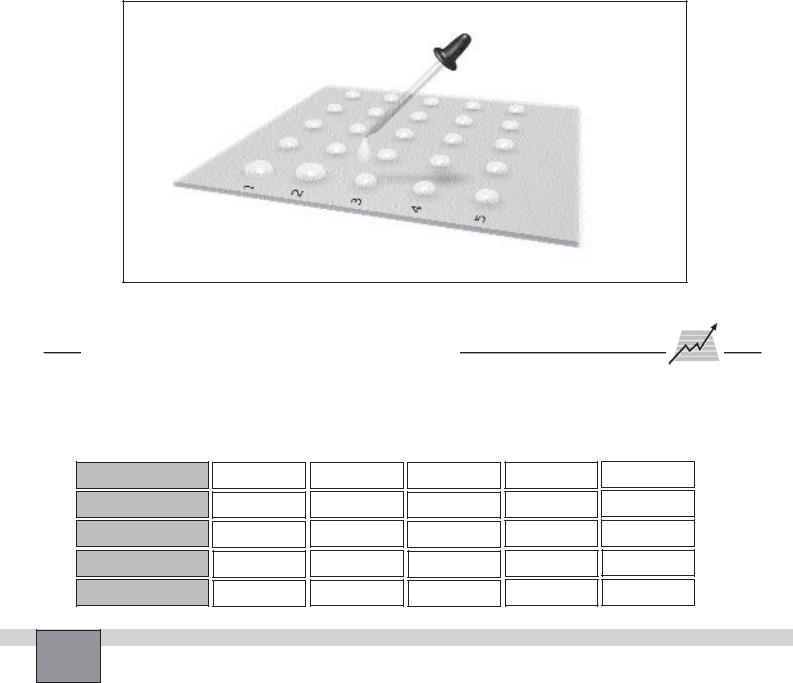
1.Place five drops of the first solution side by side with a dropper on the glass plate. Mark the line of drops as 1 with marker pen as seen in the Figure.
Note: Do not let the drops too close to each other.
2.Below the line of drops of the fist solution, place five drops of second solution side by side on the glass plate. Mark the line of drops as 2 with marker pen.
Note: Use different droppers for different solutions or clear and rinse the dropper with distilled water before using for different solution.
3.Repeat the step 2 for the other solutions.
4.Add one drop of the fifth solution on the first drops of the first, second, third, fourth and fifth solutions on the glass plate. Observe weather any precipitation occurs
Figure
or not. Write “+” sign for the mixtures that form a precipitate and “-” sign for the mixture that do not form a precipitate in the Table in “Observations and Data Tables”.
5.Add one drop of the fourth solution on the second drops of the fist, second, third, fourth and fifth solutions on the glass plate. Observe weather any precipitation occurs or not. Record your observations in Table in “Observations and Data Tables”.
6.Add one drop of the third solution on the third drops of the solutions and so on. Record your observations in the Table in “Observations and Data Tables”.
7.Add second solution on the fourth drops of the solutions and add first solution on the fifth drops of the solutions. Record your observations.
OBSERVATIONS AND DATA TABLES
1.Fill the table by writing “+” sign for the mixtures that form a precipitate and “-” sign for the mixtures that do not form a precipitate for group 1.
Temperature |
|
KI |
|
NaOH |
|
Ba(NO3)2 |
|
Pb(NO3)2 |
|
FeCl3 |
|
|
|
|
|
|
|
|
|
|
|
FeCl3
Pb(NO3)2
Ba(NO3)2
NaOH
KI
Experiment – 52 Can salt solutions react when they are mixed?
138

2.Fill the table by writing “+” sign for the mixtures that form a precipitate and “-” sign for the mixture that do not form a precipitate for group 2.
Temperature |
|
Sr(NO3)2 |
|
AlCl3 |
|
BaCl2 |
|
Na2SO4 |
|
Ba(NO3)2 |
|
|
|
|
|
|
|
|
|
|
|
Ba(NO3)2
Na2SO4
BaCl2
AlCl3
Sr(NO3)2
EVALUATIONS AND CONCLUSIONS 


1.Why some of the mixtures form precipitate but the others not? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the net ionic reaction equations for the each precipitation.
Precipitate |
|
Net ionic reaction |
|
|
|
............................. 

............................. 

............................. 

............................. 

............................. 

............................. 

............................. 

............................. 

............................. 

............................. 

Experiment – 52 Can salt solutions react when they are mixed? |
139 |
|

How do acids act on metals?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : |
To observe the behaviour of acids on metals. |
|
|
|
|
To observe the formation of salt and hydrogen gas. |
|
|
|
EQUIPMENT and MATERIALS: |
|
|
||
Equipment |
|
• Wire gauze |
(1) |
Chemicals and Other Materials |
• Test tube |
(6) |
• Burner |
(1) |
• Zinc |
• Dropper |
(2) |
|
|
• Aluminium |
• Evaporating dish |
(1) |
|
|
• Copper |
• Test tube rack |
(1) |
|
|
• Hydrochloric acid, 3 M |
• Tripod |
(1) |
|
|
• Sulphuric acid, 1 M |
PRE-LAB DISCUSSION
Metals which are much more electropositive than hydrogen, react with dilute hydrochloric acid or dilute sulphuric acid to liberate hydrogen gas. The metals e.g. Zn, Mg and Fe supply electrons to the H+ ion to form H2 gas. Metals which are
less electropositive than hydrogen, e.g. Cu, Ag and Au, do not react with hydrochloric acid. They may react with oxyacids, e.g. sulphuric and nitric acid. Such reactions do not liberate hydrogen gas.
PROCEDURE
Set-up
—Place 6 test tubes into the test tube rack and number them.
—Put a piece of zinc, aluminium and copper into the test tubes 1 to 3 and into the test tubes 4 to 6.
Figure-1
Note: Each metal should be found in two test tubes.
Procedure
1.Add some hydrogen chloride into the test tubes 1 by using dropper.
Caution: Handle any acid with great care.
Figure-2
Experiment – 53 How do acids act on metals?
140

2.Hold an empty test tube with the opening end over the test tube 1 and carry out hydrogen test for the collected gas. Record your observations in the Table in “Observations and Data Tables”.
3.Repeat step 1 and 2 for the test tubes 2 and 3, then record your observations in the Table in “Observations and Data Tables”.
4.Proceed the same way by using sulphuric acid for the test tubes from 4 to 6.
Note: Use clear dropper for the sulphuric acid.
5.Place several drops of the solution in the test tube 1 on the evaporating dish and evaporate water of the solution and record your observations in Table in “Observations and Data Tables”.
6.Repeat step 5 for the solutions from 2 to 6.
OBSERVATIONS AND DATA TABLES 


1.Fill the table with your observations on the reactions and hydrogen test.
Metal |
|
After addition of |
|
Hydrogen test |
|
After addition of |
|
Hydrogen test |
|
hydrochloric acid |
|
|
sulphuric acid |
|
|||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Zinc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aluminium |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Copper |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.Note your observations on evaporation of the resultant solutions in the test tubes. Write for the salt formation (+) sign otherwise (-) sign.
Solution 1 |
|
Solution 2 |
|
Solution 3 |
|
Solution 4 |
|
Solution 5 |
|
Solution 6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS 


1.What can you say about the products of acid and metal reactions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the formulas and the names of the salts produced in the experiment.
Test tube 1 |
: ......................................................... |
Test tube 4 |
: ......................................................... |
Test tube 2 |
: ......................................................... |
Test tube 5 |
: ......................................................... |
Test tube 3 |
: ......................................................... |
Test tube 6 |
: ......................................................... |
3.Draw a conclusion from your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 53 How do acids act on metals? |
141 |
|

How can you determine the concentration of an unknown base solution?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the molarity of a sodium hydroxide solution with a standard hydrochloric acid solution.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Buret |
(1) |
• Hydrochloric acid, 0.1M |
• Erlenmeyer flask, 250 mL |
(1) |
• Sodium hydroxide solution, Unknown |
• Dropper |
(1) |
• Phenolphthalein |
• Support base |
(1) |
|
• Support rod |
(1) |
|
• Buret clamp |
(1) |
|
• Bosshead |
(1) |
|
PRE-LAB DISCUSSION
In the laboratory, it is important to determine concentration of acid-base solutions. The reaction that takes place between acid and base solutions is called neutralisation. A neutralisation is performed by using a technique called titration in which a known volume and concentration of a solution in a buret (so called standard solution) is gradually added to a known volume of another solution until a neutral solution is obtained (end point or equivalence point). To control the neutrality of the final solution, we make use of the acid-base indicators. An acid-base indicator is a substance whose colour is sensitive to the [H3O]+ or pH of the
medium. If the solution of unknown concentration is basic, a standard acid solution is added to the base solution until it is neutralised.
When carrying out an acid-base titration, you must be able to recognise when to stop adding the standard solution, that
is, when neutralisation is reached. This is the purpose of the acid-base indicator mentioned above. A sudden change in colour of the indicator shows that neutralisation is complete. At this point, the number of hydronium ions from the acid is equal to the number of hydroxide ions coming from the base.
n |
= n – |
H+ |
OH |
This paint is called the end point of the titration. When the end point is reached, the volume of the used standard solution is carefully determined. Then, measured volumes of the two solutions and the known concentration of the standard solution can be used to calculate the concentration of the other solution as below.
MAcid x VAcid = MBase x VBase
PROCEDURE
Set-up
—Wash the buret with detergent solution.
—Rinse it first with tap water , then with distilled water thoroughly.
—Fix the buret on the support rod as seen in the Figure
and close the its stopcock.
— Fill the buret with 0.1 M hydrochloric acid.
Caution: Acids and bases are corrosive. Do not let them contact with your skin.
Experiment – 54 How can you determine the concentration of an unknown base solution?
142
