
experment book
.pdf
Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations on soap bubbles.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations in hydrogen test.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the balanced chemical equation for the experiment.
..................... + ..................... |
..................... |
+ ..................... |
2.What cause the bubbles rise into the air? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What can you deduce about the properties of the hydrogen gas by considering the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Compare the properties of hydrogen with those of oxygen.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 27 How can hydrogen gas be produced in laboratory?? |
73 |
|

How can oxygen gas be obtained in laboratory?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To obtain oxygen in laboratory and to investigate its properties.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Test tube |
(1) |
• Potassium permanganate |
• Support base |
(1) |
• Sulphur, powdered |
• Support rod |
(1) |
• Wood splint |
• Universal clamp |
(1) |
|
• Bosshead |
(1) |
|
• Burner |
(1) |
|
• Spatula |
(1) |
|
PRE-LAB DISCUSSION
Oxygen is a colourless and odourless gas. It is not only a component of air but also of other substances from which it can be released by chemical reactions. Pure oxygen can be
released from oxygen-rich compounds. Oxygen has characteristic properties, with whose help it can be identified. It enhances combustion and it have a greater density than air.
PROCEDURE
Set-up
—Place some potassium permanganate in a test tube about maximum 1 cm height by a spatula.
Caution: Do not touch potassium permanganate with your fingers because it produces brown spots on contact with skin.
—Clamp the test tube on the support rod with an angle as in the Figure-1.
—Adjust the burner to produce a colourless flame.
—Wear protective glasses.
Procedure
1.Heat the test tube cautiously with the small burner flame.
2.Pick up a wood splint with your right hand and ignite it in the flame. Allow about 0.5 cm of the splint to burn off, then blow the flame out.
3.Immediately insert the smouldering end of the splint in to the test tube and move it toward the potassium permanganate. Record your observations in the “Observations and Data Tables”.
Note: Oxygen gas can be collected in a test tube over water as described in the previous experiment.
4.Take small amount of powdered sulphur with a steel spatula. Heat until sulphur just starts to burn with burner as in the Figure-2.
5.Hold the sulphur over the potassium permanganate layer in the test tube. Record your observations in the “Observations and Data Tables”.
Experiment – 28 How can oxygen gas be obtained in laboratory?
74
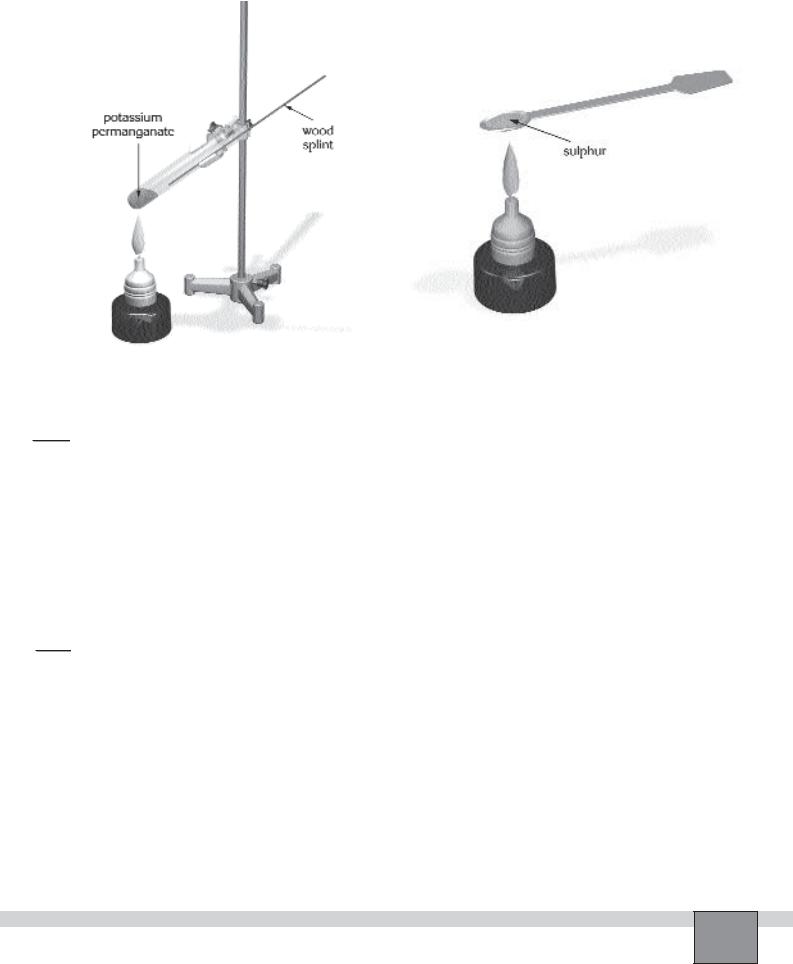
Figure-1 |
|
Figure-2 |
|
|
|
OBSERVATIONS AND DATA TABLES 


1.Note your observations on the test tube with potassium permanganate?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations when the burning sulphur inserted through the test tube.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the balanced chemical reaction equation for the formation of oxygen.
...........................................................................................................................................................................................
2.Write the balanced chemical reaction equation for the burning of sulphur.
...........................................................................................................................................................................................
3.What can you say about properties of oxygen considering the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.How do oxygen gas be produced in industry? Research.
...........................................................................................................................................................................................
Experiment – 28 How can oxygen gas be obtained in laboratory? |
75 |
|
How can metallic copper be obtained from its oxide?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To obtain copper metal from copper (II) oxide with the help of hydrogen gas.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(2) |
• Bosshead |
(1) |
• Copper(II) oxide |
• Right-angled glass tube |
(1) |
• Balance |
(1) |
• Sulphuric acid, 3 M |
• Glass tube, straight |
(1) |
• Tripod |
(1) |
• Copper sulphate solution, 1 M |
• Rubber tubing, 10 cm |
(1) |
• Burner |
(1) |
• Zinc, granulated |
• Spatula |
(1) |
• Protective glasses |
(1) |
|
• Rubber stopper, with a hole (1)
• Support base |
(2) |
• Support rod |
(2) |
• Universal clamp |
(2) |
PRE-LAB DISCUSSION
Copper is in group IB in the periodic table. It is one of the inert metals. Copper is not affected by binary acids like hydrochloric acid. On the other hand, it reacts with oxy-acids such as sulphuric acid and nitric acid. When copper is heat-
ed in air, it is covered by copper oxide which is fairly resistant to corrosion. Copper ion in the copper oxide can be reduced to copper metal by hydrogen gas.
PROCEDURE
Set-up
—Place about 0.5 g of copper(II) oxide (or a spatula) in a test tube.
—Fill another test tube with 10-15 mL of 3M Sulphuric acid solution. Add a few drops of copper sulphate solution.
Note: Addition of copper sulphate speeds up the reaction.
— Set up the apparatus as seen the Figure.
Note: Lubricate glass-rubber connections with glycerol.
— Wear protective glasses.
Procedure
1.Place a few pieces of granulated zinc into the test tube which contains acid solution, then immediately assemble the apparatus.
2.Allow all the air in the test tube to displace with hydrogen gas for 30-60 seconds.
Caution: do not heat until all air is displaced with hydrogen, otherwise hydrogen may explode.
3.While the gas is coming onto copper(II) oxide, heat the test tube which contains copper oxide gently with the burner flame about 3 cm high.
4.Heat strongly until the red copper metal is formed.
5.When the reduction is complete, remove the burner and leave the apparatus to cool. Note your observations in the “Observations and Data Tables”.
Note: It is important to keep a small flow of hydrogen gas over the copper metal until it is quite cold. Otherwise air will enter and oxidise the copper.
Caution: Do not inhale or swallow copper compounds because they are hazardous to health.
Experiment – 29 How can metallic copper be obtained from its oxide?
76
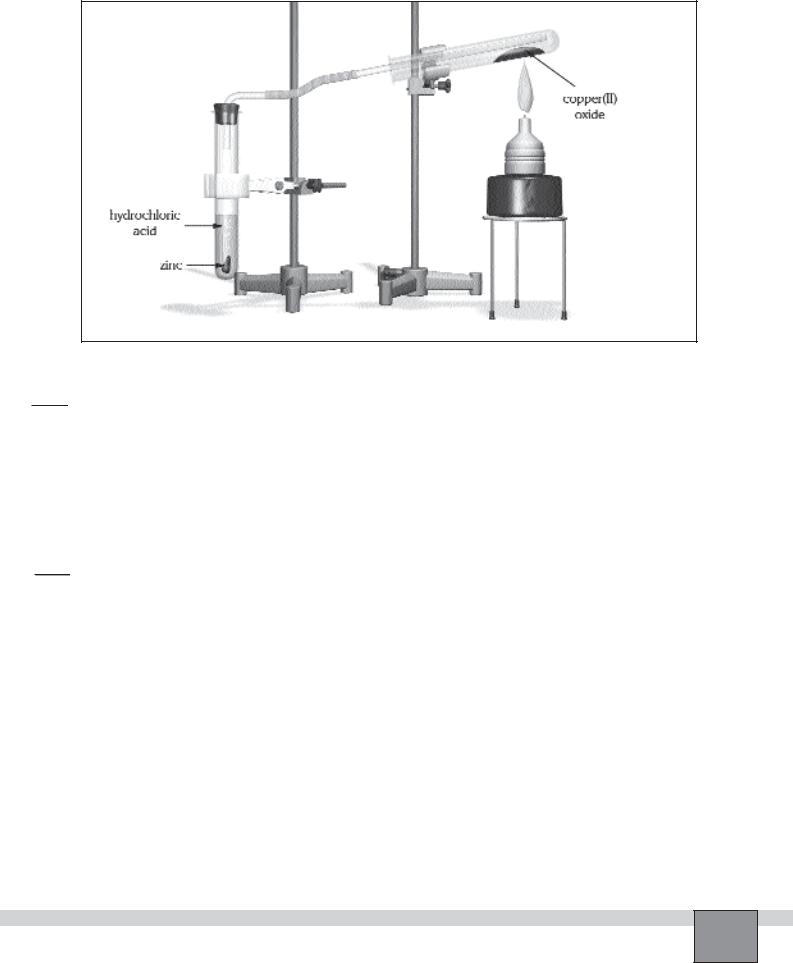
Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the balanced reaction equation for the formation of copper.
...........................................................................................................................................................................................
2.How can you deduce that the reddish compound is copper? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Which property of hydrogen make the reaction possible? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Explain the terms below and give at least two examples for each.
a)Reducing agent: ............................................................................................................................................................
b)Oxidising agent: ............................................................................................................................................................
Experiment – 29 How can metallic copper be obtained from its oxide? |
77 |
|
Preparation of carbon dioxide and its properties
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare carbon dioxide in laboratory and to investigate its properties.
EQUIPMENT and MATERIALS:
Equipment |
|
• Support base |
(1) |
Chemicals and Other materials |
• Separatory funnel |
(1) |
• Support rod |
(1) |
• Calcium carbonate |
• Erlenmeyer |
(1) |
• Universal clamp |
(2) |
• Hydrochloric acid, 10% |
• Beaker, 500 mL |
(1) |
• Bosshead |
(2) |
• Lime water, (calcium hydroxide solu- |
• Beaker, 200 mL long |
(1) |
• Rubber stopper, with two holes |
(1) |
tion) |
• Beaker, 150 mL |
(1) |
• Rubber tubing, 10 cm |
(1) |
• Candle, (three different long) |
• Test tube |
(1) |
• Spatula |
(1) |
|
• Right - angled glass tube |
(2) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Carbon dioxide is a colourless gas and it smothers combustion like nitrogen. It have a considerably higher density than air. Carbon dioxide can be obtained in laboratory from carbonates by adding acids.
CaCO3 + 2HCl CaCl2 + CO2 + H2O
Carbon dioxide is an acidic oxide and it is known as unhydrous acid. It acts an acid against bases.
CO2 + NaOH Na2CO3 + H2O
Carbon dioxide is slightly soluble in water.
PROCEDURE
Set-up
— Fill two third of the test tube with lime water.
Note: Use clear supernatant solution.
Note: If you prepare lime water yourself, begin early and allow the undissolved calcium hydroxide to settle down.
—Place two spatulas of calcium carbonate into the erlenmeyer flask.
—Insert the separatory funnel into one of the rubber stoppers’s holes and the right-angled glass tube into the other hole.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
—Attach about 20 cm rubber tube to the right-angled glass tube and insert the opening end of the tube into lime water in the test tube.
—Set the apparatus as seen in the Figure-1
— Wear protective glasses.
Procedure:
1.Close the stopcock of the separatory funnel and fill it with dilute hydrochloric acid about half full.
2.Open the stopcock of the separatory funnel slowly and allow several drops of hydrochloric acid to drop onto the calcium carbonate, then close the stopcock.
Note: Carefully add hydrochloric acid dropwise, otherwise the reaction is too violent.
3.Repeat the step 2 until gas production is complete. Record your observations in the “Observations and Data Tables”.
Caution: lime water and hydrochloric acid cause burns.
4.Remove lime water in the test tube and clean it. Fill the half of the test tube with distilled water. Repeat the steps from 1 to 3 with fresh calcium carbonate.
Experiment – 30 Preparation of carbon dioxide and its properties
78

5.Examine the acidity of the water with litmus paper. Record your observations in the “Observations and Data Tables”.
Note: To observe acidity of water, several drops of methyl red indicator can be added into water.
6.Put a clean 400 mL beaker on the bench. Place the three candles on the bottom of the beaker with the help of drops of wax as in the Figure-2.
7.Place the opening of the rubber tubing on the bottom of the beaker and light the candles.
8.Repeat the steps from 1 to 3 and record your observations in the “Observations and Data Tables”.
Figure-1 |
Figure-2 |
|
|
|
|
9.Place the smallest candle in a 150 mL beaker and light it.
10.Place the opening of the rubber tubing on the bottom of the another 200 mL beaker. Fill the beaker with carbon dioxide by repeating the steps from 1 to 3.
Note: Be sure there is no air flow over the beaker, otherwise carbon dioxide diffuses away.
11.Pour the carbon dioxide cautiously like a liquid into the 150 mL beaker the burning candle. Record your observations in the “Observations and Data Tables”.
Note: The beaker filled with carbon dioxide must be moved carefully, otherwise it diffuses away.
OBSERVATIONS AND DATA TABLES 


1.Note your observations when hydrochloric acid is added on the calcium carbonate?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations when produced carbon dioxide passes through water.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Note your observations on the candles when carbon dioxide is produced?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Note your observations on the candle smallest when carbon dioxide is poured on it.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 30 Preparation of carbon dioxide and its properties |
79 |
|

EVALUATIONS AND CONCLUSIONS 


1.Based on the experiment, list several properties of the produced carbon dioxide.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the chemical reaction for the test of the carbon dioxide.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.When carbon dioxide is passed through lime water, first turbidity is formed and then disappears. Explain Why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What is the colour of litmus paper, when carbon dioxide is passed through the water? Explain why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Why does the candles blow out, when the beaker starts to fill with carbon dioxide gas?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
6.What makes the carbon dioxide to be poured into the beaker like liquid?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
7.List several occurrences of carbon dioxide in natura. How could you the test that it contains carbon dioxide?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
8.List several usages of the carbon dioxide. Explain which properties make carbon dioxide be useful in that area?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 30 Preparation of carbon dioxide and its properties
80
How can ammonia be obtained in laboratory?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare ammonia in the laboratory and to investigate the properties of ammonia.
EQUIPMENT and MATERIALS:
Equipment |
|
• Bosshead |
(1) |
• Sodium hydroxide, pellets |
• Test tube |
(2) |
• Burner |
(1) |
• Sulphuric acid, 1M |
• Dropper |
(1) |
• Spatula |
(1) |
• Phenolphthalein indicator |
• Support base |
(1) |
Chemicals and Other materials |
• Support rod |
(1) |
• Ammonium chloride (or household |
• Universal clamp |
(1) |
detergent), 1M |
•Iron(III) chloride crystals
•Distilled water
•Red litmus paper
PRE-LAB DISCUSSION
In laboratory ammonia is prepared by heating ammonium chloride and calcium hydroxide.
2NH4Cl(s) + Ca(OH)2(s) 2H2O(l) + NH3(g) + CaCl2(s)
Molar mass of ammonia is 17 grams. Ammonia is a colourless gas with a sharp smell and less dense than air. It is very soluble in water (in 1 litre water, 700 litres of ammonia can be soluble). This solution is called ammonia water or liquid ammonia.
At high pressure ammonia liquifies. The liquid ammonia is alkaline. It is not a strong base. It produces OH– ion in water. In
the reactions of ammonia, oxidation states of nitrogen change.
NH |
|
|
|
|
|
|
|
NH + + OH |
_ |
3 |
+ H |
O |
|
NH |
OH |
|
pH > 7 |
||
|
|
||||||||
|
|
||||||||
|
2 |
|
4 |
|
4 |
|
|||
In an ammonia molecule, nitrogen has a free electron pair and hydrogen ion has an empty s-orbital. Non-bonding electron pair of nitrogen combines with free s-orbital of hydrogen ion and a fourth covalent bond is formed by donor-acceptor mechanism. Nitrogen atom is the donor of the shared elec- tron-pair and hydrogen ion is the acceptor.
|
PROCEDURE |
Set-up |
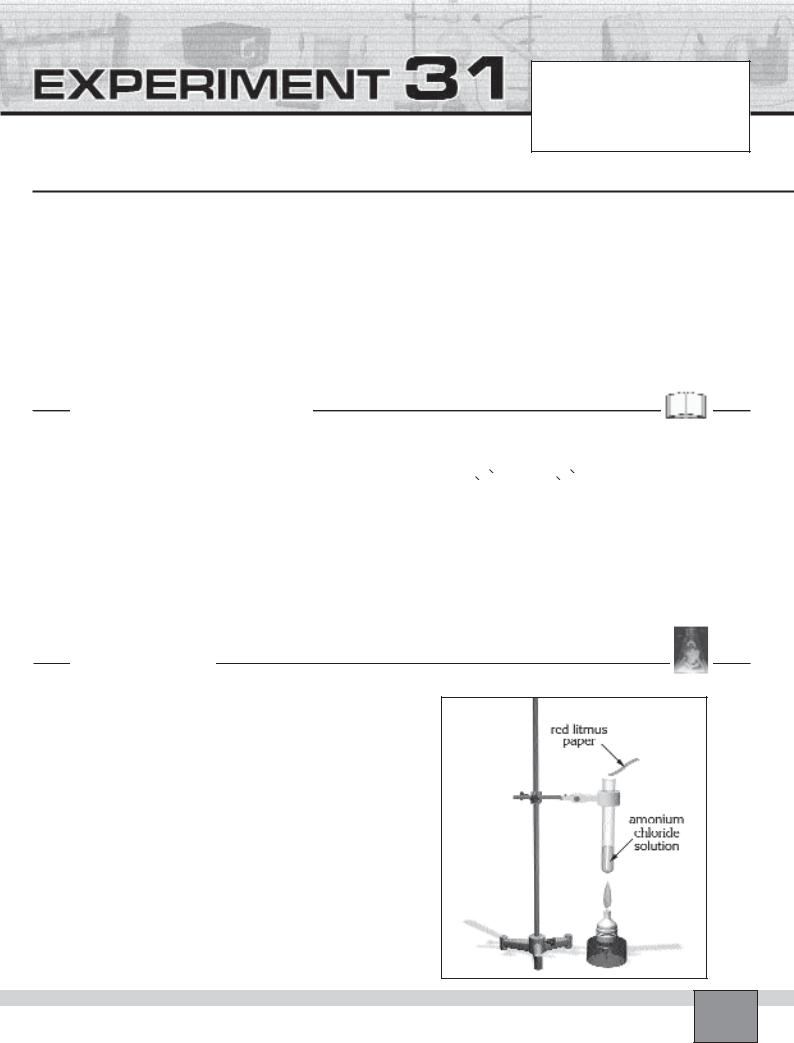
Figure |
—Fill one of the test tubes about 2 cm high with ammonium chloride solution, then clamp it as seen in the Figure.
—Take a red litmus paper and moisten it with distilled water.
Procedure:
1.Put 1-2 sodium hydroxide pellets into the ammonium chloride solution with a spatula.
Caution: Since sodium hydroxide is highly corrosive, do not touch, it bare fingers.
2.While heating the solution slowly with burner, first hold dry red litmus paper then the moist red litmus paper over the opening end of the test tube which contains ammonium chloride solution. Record your observations in “Observations and Data Tables”.
Experiment – 31 How can ammonia be obtained in laboratory? |
81 |
|

Note: The solution can be heated to get more ammonium gas.
3.Divide the obtained solution into two test tubes.
4.Add first 1-2 drops of phenolphthalein indicator with dropper, then add 1M sulphuric acid solution slowly into the one of the test tube. Record your observations in “Observations and Data Tables”.
5. Add several iron(III) chloride crystals into the other test
tube. Record your observations in the “Observations and Data Tables”.
Note: Since ammonia produces unpleasant smelling, air the room after experiment.
OBSERVATIONS AND DATA TABLES
1.Note your observations on the addition of sodium pellets onto the ammonium chloride solution.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations in the step 4 and fill the table below.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
|
|
Before addition of |
|
After addition of |
|
After addition of |
|
|
ammonium solution |
|
ammonium solution |
|
sulphuric acid solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Colour of phenolphthalein |
|
|
|
....................................... |
|
....................................... |
|
|
........................................ |
|
|
3.Note your observations when iron(III) chloride is added.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.What can be deduced about the properties of the ammonia based on the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Why the colour of the moist litmus paper changed, but that of the dry one did not? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the balanced reaction equations for the processes that take place in the experiment.
a)In step 1: .......................................................................................................................................................................
b)In step 4: .......................................................................................................................................................................
c)In step 5: .......................................................................................................................................................................
4.List several usages of the ammonia in industry and in daily life and explain which properties make it useful in that area.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 31 How can ammonia be obtained in laboratory?
82
