
experment book
.pdf
EVALUATIONS AND CONCLUSIONS 


1.Compare the solubility of the substances in water in increasing order?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Can solubility always be distinctive property for all liquids in water?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Which properties of the liquids affect the solubility in water? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 5 Do all liquids dissolve in water? |
23 |
|
Do gases dissolve in water in the same ratio?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the solubility of gases in water.
EQUIPMENT and MATERIALS:
Equipment |
|
• Test tubes |
(2) |
• Support base |
(2) |
• Support rod |
(2) |
• Universal clamp |
(4) |
• Bosshead |
(4) |
• Crystallising dish |
(1) |
• Glass tube, straight |
(1) |
• Burner |
(1) |
• Graduated cylinder, 10 mL |
(1) |
• Rubber stopper, with one hole (1)
• Rubber stopper, without hole (2)
Chemicals and Other Materials
•Ammonia
•Water
PRE-LAB DISCUSSION
A solute is any substance dissolved in a solvent. It might be a gas. For example, beverages include dissolved carbon
dioxide. Sea water contains dissolved oxygen gas besides many other gases. Solubility of gases in water is different.
PROCEDURE
Set-up
—Lubricate the stopper-glass tube connection with glycerol. Then insert the glass tube into the hole of the rubber stopper.
—Put 5 mL ammonia into a test tube. Close the test tube with the stopper which connected glass tube.
Note: Do not taste or inhale ammonia.
—Insert the opening end of the glass tube into another test tube and fix them on the stand as in the Figure-1.
Procedure
1.Heat the ammonia solution for 2-3 minutes. Let the ammonia gas fill into upper test tube. Extinguish the burner flame.
Note: Do not heat the ammonia too much. It’s smell may diffuse
throughout the room. It has unpleasant smell.
2.Close the mouth of upper test tube with a stopper without turning up. Then sink it into the water in crystallising dish as seen in Figure-2.
3.Take another empty test tube and close the mouth of it with rubber stopper. Then sink it into the water in the Crystallising dish as seen in Figure-2
4.Open the mouth of the both test tubes in the water and wait for 10 minutes.
5.Compare the levels of the water in the test tubes and record your observations in the Table in “Observations and Data Tables”.
Caution: Air the room after experiment because of the unpleasant smell of ammonia.
Experiment – 6 Do gases dissolve in water in the same ratio?
24

Figure-1 |
|
Figure-2 |
|
|
|
OBSERVATIONS AND DATA TABLES
1.Note your observations in the Table below.
Solubility of gases in water (low or high).
Ammonia |
|
Air |
|
|
|
|
|
|
EVALUATIONS AND CONCLUSIONS
1.Compare the solubility of the gases in water in increasing order?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Can solubility of gases in water be distinctive property for all gases? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Which property of the ammonia affects it’s solubility in water? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 6 Do gases dissolve in water in the same ratio? |
25 |
|

How does the temperature affect the solubility of salts in water?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the effect of the temperature on the solubility of salts in water.
EQUIPMENT and MATERIALS:
Equipment |
|
• Wire gauze |
(1) |
Chemicals and Other Materials |
• Test tubes |
(2) |
• Balance |
(1) |
• Sodium chloride |
• Test tube rack |
(1) |
• Support base |
(1) |
• Potassium nitrate |
• Beaker, 250 mL |
(1) |
• Support rod |
(1) |
• Water |
• Graduated cylinder, 10 mL |
(1) |
• Universal clamp |
(2) |
• Marker pen |
• Thermometer |
(1) |
• Bosshead |
(2) |
|
• Burner |
(1) |
• Rubber stopper, without hole |
(1) |
|
• Tripod |
(1) |
• Rubber stopper, with a hole |
(1) |
|
PRE-LAB DISCUSSION
Some factors affect the solubility of solids in water. One of them is temperature. In general, solubility of solids in water increases with increasing temperature.
On the other hand, solubility of some solids decreases with increasing temperature.
PROCEDURE
Set-up
—Place two test tubes into the test tube rack. Add 5 mL of water in each test tube with 10 mL graduated cylinder.
—Weigh 3 g of each of sodium chloride and, potassium nitrate salts.
—Put sodium chloride in the first test tube and potassium nitrate in the second test tube. Then label the tubes with marker pen.
—Close the test tubes with the rubber stoppers and shake them vigorously for several minutes. Place them into the 250 mL beaker which contains water as in the Figure.
—Place a thermometer into one of the tubes as in the Figure.
Note: Do not let the test tubes and thermometer contact with the bottom of the beaker.
—Be sure that water level in the beaker is over the level of the solutions in the test tubes.
—Wait for awhile to make the temperature of the system equal.
Procedure
1.Ignite the burner and heat the test tubes in water up to 50oC.
2.Extinguish the burner flame. Observe if there is any solid salts in the test tubes. Record your observations in the Table in “Observations and Data Tables”.
Figure
Experiment – 7 How does the temperature affect the solubility of salts in water?
26

OBSERVATIONS AND DATA TABLES 


1.Note your observations on effect of temperature on solubility of solids below.
Effect of temperature on solubility (increase or decrease solubility with temperature)
Sodium chloride |
|
Potassium nitrate |
|
|
|
........................................................ ........................................................
EVALUATIONS AND CONCLUSIONS 


1.How does increase in temperature affect the solubility of salts?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Give a method to change crystallised honey into its original liquid form.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.List the most soluble solids, liquids and gases in water at room temperature? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Does change in temperature affect the solubility of all substances in the same manner? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 7 How does the temperature affect the solubility of salts in water? |
27 |
|

How can a supersaturated solution be prepared?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare a supersaturated solution with sodium thiosulfate in water.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tubes |
(1) |
• Burner |
(1) |
• Sodium thiosulphate |
• Beaker, 250 mL |
(2) |
• Tripod |
(1) |
|
• Graduated cylinder, 10 mL |
(1) |
• Wire gauze |
(1) |
|
• Test tube holder |
(1) |
• Rubber stopper, without hole |
(1) |
|
PRE-LAB DISCUSSION
A solution which contains more solute than a saturated solution at the same temperature is said to be super saturated solution.
Such solutions are generally unstable solutions. The excess dissolved salt in a supersaturated solution precipitates immediately with addition of a crystal or by shaking the solution.
PROCEDURE
Set-up |
Figure |
|
—Weigh about 6 g of sodium thiosulphate and pour it into a test tube. Add 2 mL of water with 10 mL graduated cylinder and close the test tube with a rubber stopper.
—Place the test tube in the beaker with water as seen in the Figure.
Procedure
1.Ignite the burner and heat the test tube by heating the water in the beaker until all sodium thiosulphate has dissolved.
2.Pick up the test tube with test tube holder, and put it without shaking into a beaker that contains cold water.
3.Allow the solution to cool, gradually.
4.After the solution in the test tube cooled down, put a small crystals of sodium thiosulphate into the test tube. Record your observations in “Observations and Data Tables”.
Note: Put the produced salts in the disposal box to use later.
Experiment – 8 How can a supersaturated solution be prepared?
28
OBSERVATIONS AND DATA TABLES 


1.Note Your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Can a saturated water solution be prepared for all salts? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How do saturated solutions be used in industry and in daily life? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 8 How can a supersaturated solution be prepared? |
29 |
|

How can iron be separated from the mixture of iron and sulphur?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate iron from the mixture of iron and sulphur powder with the help of a magnet. To show that the characteristic properties of substances do not change in a mixture.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Magnet |
(1) |
• Iron fillings |
• Stirring rod |
(1) |
• Sulphur powder |
• Spatula |
(1) |
• Sheet of paper |
PRE-LAB DISCUSSION
Mixtures consist of two or more pure substances. Therefore, they are not pure substances. Unlike pure substances, they have variable composition. They can be separated by simple physical methods.
There are two types of mixtures; Homogeneous and Heterogeneous.
- Homogeneous mixture: It has the same properties (e.g. density and composition) in each part of the mixture. For example: Water-Salt, and Water-Alcohol mixtures.
-Heterogeneous mixture: It has not the same properties in all parts of the mixture.
PROCEDURE
Set-up |
Figure |
—Place a piece of paper on the bench then put a spoon full of iron filings and a spoon full of sulphur powder on the paper.
—Mix them with a stirring rod and examine the mixture with naked eyes. Record your observations in “Observations and Data Tables”.
Procedure
1.Hold the magnet over the mixture and see that one of the components is picked up, but the other is not. Note your observations in “Observations and Data tables”.
Note: Wrapping the magnet in paper after using will help to remove iron filings easy.
Experiment – 9 How can iron be separated from the mixture of iron and sulphur?
30

OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of matter is used for the separation of the iron and sulphur mixture? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Can you give alternative method for the separation of the iron and sulphur mixture?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Research, which other matter can be separated by the method used in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4. Is the mixture in the experiment homogeneous or heterogeneous? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Which characteristic properties of iron and sulphur are observed in the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
6.Did the characteristic properties of iron and sulphur change in the mixture?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 9 How can iron be separated from the mixture of iron and sulphur? |
31 |
|
Can an electrified object be used to separate mixtures?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate pepper from the mixture of salt and red pepper with the help of an electrified object.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 100 mL |
(1) |
• Red pepper, dry |
• Stirring rod |
(1) |
• Table salt |
• Spatula |
(1) |
• Sheet of paper |
• Ebonite rod, (or comb) |
(1) |
|
PRE-LAB DISCUSSION
Mixtures can be separated by physical methods. One of the methods is using electrified objects. When an electrified ob-
ject is approached another object which has free electrons on its surface, the electrified object attracts the other one.
PROCEDURE
Set-up |
Figure |
|
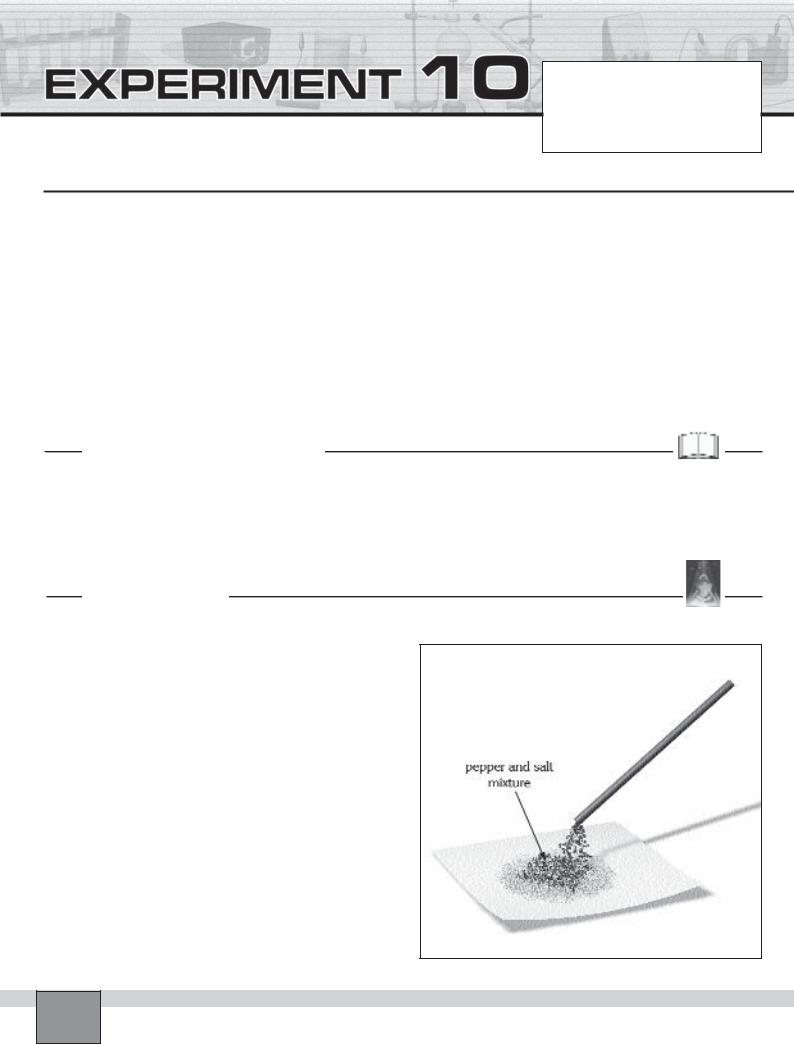
—Place 100 mL beaker on the bench then put a spoon full of dry table salt and a spoon full of dry red pepper into the beaker.
Note: The pepper must be dry. Otherwise electrified object does not attract them.
— Mix the substances well with a stirring rod.
Note: Prefer the salt as coarse.
—Take some of the mixture on a piece of paper with a spatula.
Procedure
1.Rub the ebonite rod against the woollen cloth or hair. Then hold the electrified end of the ebonite rod over the salt-pepper mixture.
Note: Do not let the ebonite rod to get in contact with the mixture.
2.Transfer the component attracted by the ebonite rod on another piece of paper. Note your observations in “Observations and Data tables”.
Experiment – 10 Can an electrified object be used to separate mixtures?
32
