
experment book
.pdf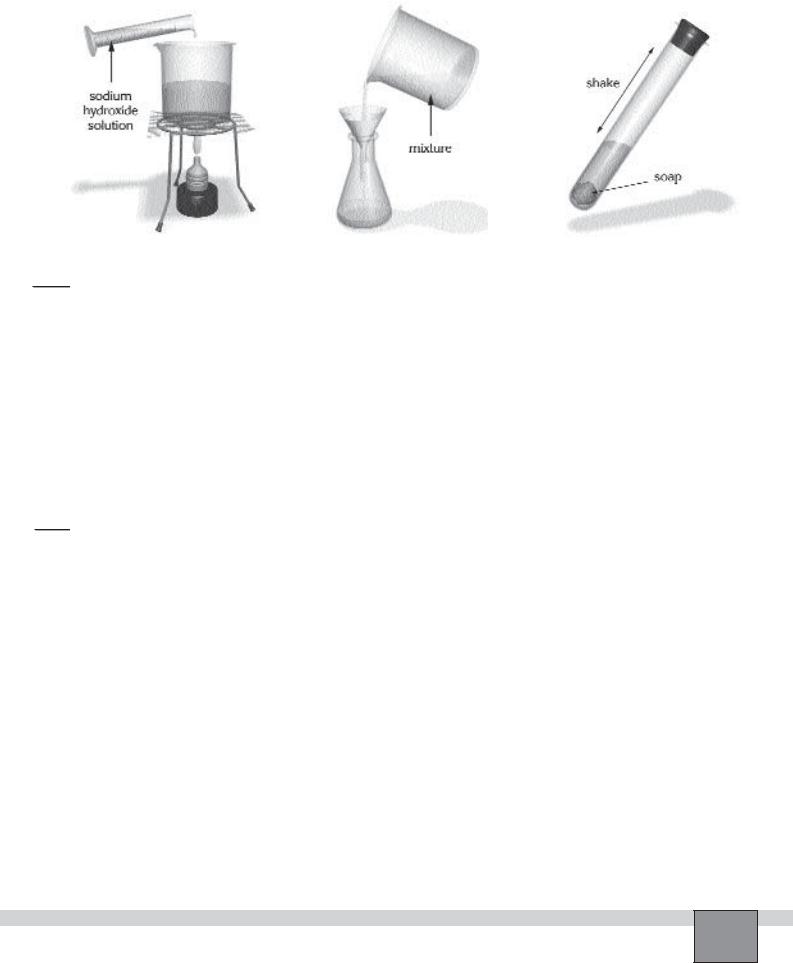
Figure-1 |
|
Figure-2 |
|
Figure-3 |
|
|
|
|
|
|
|
|
|
|
OBSERVATIONS AND DATA TABLES 


1.Note your observations on addition of sodium hydroxide.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on testing of your product with distilled water.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw conclusions from your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the function of the salt solution? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Formulate the equation for the reactions in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Why did you rinse your product with distilled water? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.List the fats and oils which are used in the production of soaps found in your home. Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 76 How can soap be produced? |
193 |
|
What are the glucose, sucrose and lactose?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the reducing action of glucose, sucrose and lactose and to hydrolyse sucrose.
EQUIPMENT and MATERIALS:
Equipment |
|
• Spatula |
(1) |
• Lactose |
• Beaker, 250 mL |
(1) |
• Burner |
(1) |
• Hydrochloric acid, 0.1 M |
• Beaker, 100 mL |
(3) |
•Tripod |
(1) |
• Sodium hydroxide, 0.1 M |
• Graduated cylinder, 10 mL |
(1) |
• Wire gauze |
(1) |
• Tollens’ reagent |
• Test Tube |
(3) |
Chemicals and Other Materials |
|
• Benedict’s reagent |
• Dropper |
(1) |
• Glucose |
|
• Distilled water |
• Stirring rod |
(1) |
• Sucrose |
|
• Litmus paper |
PRE-LAB DISCUSSION
Ordinary sugar is a disaccharide called sucrose. Sucrose, the most widely occurring disaccharide, is found in all photosynthetic plants and is obtained commercially from sugar beets and sugar canes. The molecular formula of sucrose is
C12H22O11. When sucrose is hydrolysed in acid or base, glu-
cose and fructose are formed. Sucrose give negative tests with Benedict’s and Tollen’s reagents.
|
PROCEDURE |
Set-up |
Figure |
—Place three test tubes into the test tube rack and number them with marker pan.
—Put 5 mL of glucose solution in to test tube 1.
—Put 5 mL of sucrose solution in to test tube 2.
—Put 5 mL of lactose solution in to test tube 3.
Preparation of solutions: Place three 100 mL beakers on the bench and put a tip spatula of glucose, sucrose and lactose into the beaker separately. Dissolve them in 25 mL distilled water with stirring.
—Fill the two third of 250 mL beaker with tap water and place it on the wire gauze on the tripod.
Procedure
1.Add Tollens’ reagent into the test tube 1, 2 and 3. Observe that a silver mirror or precipitate is formed and record your observations in “Observations and Data Tables”.
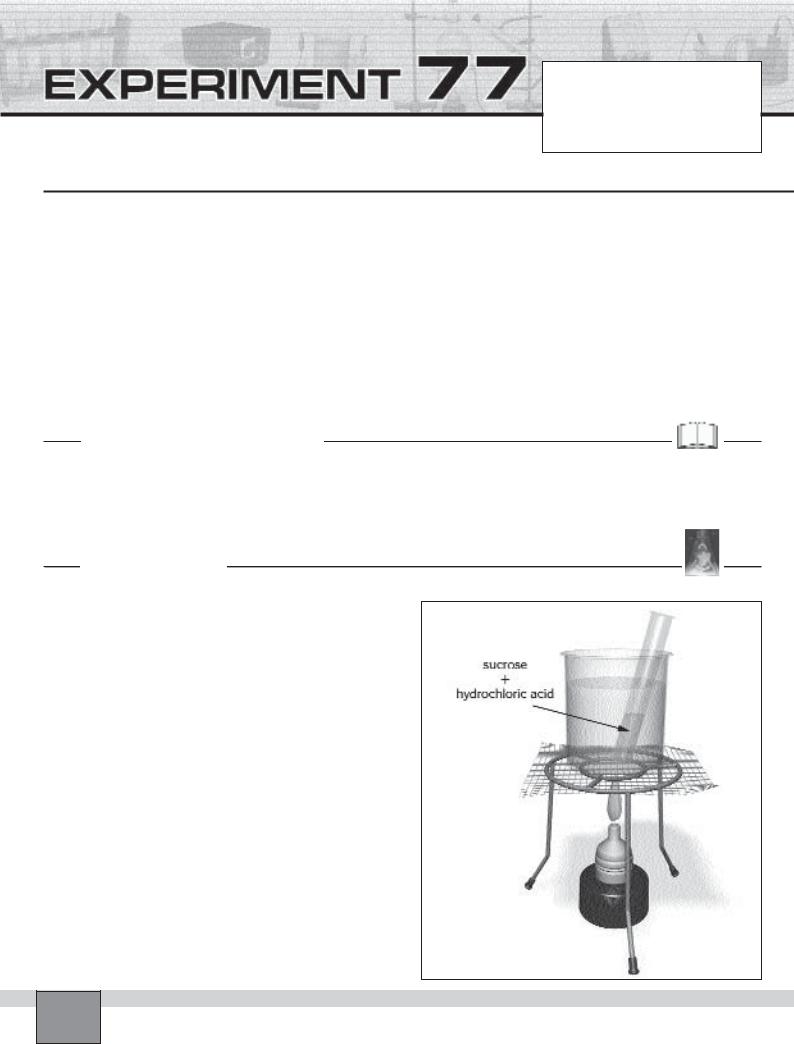
2.Put 10 mL of sucrose solution into a test tube and add 1 mL 0.1 M hydrochloric acid.
3.Ignite the burner and boil the water in the beaker. Place the test tube in the beaker and heat the contents of the test tube in boiling water for 30 minutes as in the Figure.
Experiment – 77 What are the glucose, sucrose and lactose?
194

4.Extinguish the burner flame. Take the test tube out from the beaker and place it into the test tube rack. Allow the solution to cool.
5.Neutralise the solution by addition of 0.1 M sodium hydroxide solution drop by drop. Test with litmus paper.
6.Add a few drops of Benedict’s reagent into test tube with dropper and record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations on Tollens’ reagent test for;
Glucose : ......................................................................................................................................................................
...........................................................................................................................................................................................
Sucrose : ......................................................................................................................................................................
...........................................................................................................................................................................................
Lactose : ......................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on Benedict’s reagent test for sucrose.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw a conclusion from your observations?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the equations for the reactions that take place in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Classify the substances as reducing and non-reducing.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What are the functions of Tollens’ and Benedict’s reagents? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 77 What are the glucose, sucrose and lactose? |
195 |
|

How are polysaccarides hydrolysed?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the hydrolysis of starch.
EQUIPMENT and MATERIALS:
Equipment |
|
• Burner |
(1) |
• Sodium hydroxide, 0.1 M |
• Beaker, 250 mL |
(1) |
•Tripod |
(1) |
• Iodine-potassium iodide solution |
• Graduated cylinder, 10 mL |
(1) |
• Wire gauze |
(1) |
• Tollens’ reagent |
• Test Tube |
(4) |
|
|
• Benedict’s reagent |
• Dropper |
(1) |
Chemicals and Other Materials |
|
• Litmus paper |
• Stirring rod |
(1) |
• Starch |
|
• Distilled water |
• Spatula |
(1) |
• Hydrochloric acid, 0.1 M |
|
|
PRE-LAB DISCUSSION
Starch is the most important polysaccharide. It occurs in the roots, and seeds of plants. Corn, potatoes, wheat and rice are the important commercial sources of starch. It is made
up of glucose units. Starch does not reduce Benedict’s solution (Cu2+). A characteristic reaction of starch is the formation of a blue compound with iodine.
PROCEDURE
Set-up |
Figure |
—Place three test tubes into the test tube rack and number them with marker pan. Put 5 mL of fresh starch solution into two of the test tubes and 10 mL of fresh starch solution into the third test tube.
Preparation of starch solution: Add 1 g of starch into 10 mL of distilled water and stir the mixture to get smooth suspension. Add this suspension solution slowly into 120 mL of boiling distilled water with constant stirring. After addition is completed, boil the solution for 2 minutes , then allow the solution to cool.
—Fill the two third of 250 mL beaker with tap water and place it on the wire gauze on the tripod.
Procedure
1.Add a few drops of Benedict’s’ reagent into one of the test tubes and record your observations in “Observations and Data Tables”.
2.Add 1 drop of iodine-potassium iodide solution into the second tube and record your observations in “Observations and Data Tables”.
3.Add 1 mL 0.1 M hydrochloric acid into the third test tube.
4.Ignite the burner and boil the water in the beaker. Place the test tube in the beaker and heat the contents of the test tube in boiling water for 30 minutes.
Experiment – 78 How are polysaccarides hydrolysed?
196

5.Extinguish the burner flame. Take the test tube out from the beaker and place it into the test tube rack. Allow the solution to cool.
6.Neutralise the solution by addition of 0.1 M sodium hydroxide solution drop by drop. Test the acidity of the solution with litmus paper.
7.Pour the half of the solution in into another test tube.
8.Add a few drops of Benedict’s reagent into one of the test tube and record your observations in “Observations and Data Tables”.
9.Add 1 drop of iodine-potassium iodide solution into one of the tubes and record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES
1.Note your observations before hydrolysis of starch;
Benedict’s reagent test : ............................................................................................................................................
...........................................................................................................................................................................................
Iodine-potassium iodide solution : ...............................................................................................................................
...........................................................................................................................................................................................
2.Note your observations after hydrolysis of starch;
Benedict’s reagent test : ............................................................................................................................................
...........................................................................................................................................................................................
Iodine-potassium iodide solution : ...............................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw conclusions from your observations?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the equations for the reactions that take place in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 78 How are polysaccarides hydrolysed? |
197 |
|
