
experment book
.pdf
EVALUATIONS AND CONCLUSIONS 


1.Compare your result with the results of the other groups.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What can you say about the source of the error? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Explain the following terms
Molar heat of formation : ............................................................................................................................................
...........................................................................................................................................................................................
Molar heat of neutralisation : ...........................................................................................................................................
...........................................................................................................................................................................................
Heat of reaction : ............................................................................................................................................
...........................................................................................................................................................................................
4.How is energy stored in compound? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 43 How can the heat of combustion of a liquid be determined? |
113 |
|
Heat of reaction and Hess’s law
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine heat of a reaction and to prove Hess’s law.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
|
• Beaker, 100 mL |
(3) |
• Hydrochloric acid solution, 1 M |
|
• Thermometer |
(1) |
• Hydrochloric acid solution, 0.5 M |
|
• Graduated cylinder, 50 mL |
(1) |
• Sodium hydroxide solution, 1 M |
|
• |
Protective glasses |
(1) |
• Sodium hydroxide, pellets |
• |
Balance |
(1) |
• Distilled water |
PRE-LAB DISCUSSION
The energy involved in a chemical reaction is expressed in terms of the amount of heat released or absorbed during a reaction. This heat of reaction ( H) is measured in kilojoule per mole. H of an exothermic reaction (heat released) has minus sign: H of an endothermic reaction (heat absorbed) has plus sign. In this experiment you will study three related exothermic reactions involving sodium hydroxide. In the first reaction solid sodium hydroxide will dissociate into water. The heat produced in this reaction ( H1) is called the heat
of dissolution of NaOH. In the second reaction an aqueous solution of NaOH will react with an aqueous solution of HCl.
The heat of this reaction ( H2) is called the heat of neutral-
isation. In the third reaction solid NaOH will react with an aqueous solution of hydrochloric acid. This reaction is actually combination of the first and second reaction. The solid NaOH will dissociate into ions then neutralised by the acid. Thus the heat of this reaction ( H3) could be equal to
( H1 + H2). Using the calculation based on data collect-
ed in this experiment you will attempt to verify the additive nature of heat of reactions.
PROCEDURE
Set-up
—Put three 100 mL beakers on the bench and label them.
—Weigh the beakers and record their masses in the Table in “Observations and Data Tables”
—By using the graduated cylinder,
—Place 100 mL of distilled water in the beaker 1.
—Place 50 mL 1M hydrochloric acid in the beaker 2.
—Place 100 mL 0.5M hydrochloric acid in the beaker 3.
Caution: Handle acid and bases carefully to avoid any burns.
—Allow the solutions to rest until their temperature reach the room temperature. Record the temperature of the solutions in the Table in “Observations and Data Tables”.
Note: Using a polystyrene beaker instead of glass beaker increases the efficiency of the experiment.
Or cover the glass beaker with polystyrene or with other materials which isolate heat.
— Wear protective glasses.
Procedure
1.Weigh about 2 g of sodium hydroxide pellets and record it in the Table in “Observations and Data Tables”.
Caution: Do not handle sodium hydroxide with your hand since it is very corrosive.
Note: Weigh the sodium hydroxide just before adding into the water. Otherwise, it starts to be liquid gradually. Because it absorbs the water vapour in the air.
Experiment – 44 Heat of reaction and Hess’s law
114

2. Add the sodium hydroxide pellets to the water in the |
Figure |
beaker 1, then stir the mixture by using thermometer |
|
|
|
until all sodium hydroxide has dissolved and increase |
|
in temperature has stopped. |
|
Note: Make sure that there is no air flow around the set.
3.Read the highest temperature reached and record it in the Table in “Observations and Data Tables”.
4.Measure exactly 50 mL of 1M sodium hydroxide solution with graduated cylinder
Note: Before addition, Allow the sodium hydroxide solution to rest until its temperature reaches the room temperature.
5.Add sodium hydroxide solution to hydrochloric acid solution in the beaker 2.
6.Stir the mixture with the thermometer until increase in temperature has stopped.
7.Read the highest temperature reached and record it in the Table in “Observations and Data Tables”.
8.Weigh sodium hydroxide pellets as the same amount as in the step 1.
Note: Weigh the sodium hydroxide just before adding into the water. Otherwise, it starts to be liquid gradually. Because it absorbs the water vapour in the air.
9.Add the sodium hydroxide pellets to hydrochloric acid solution in the beaker 3, then stir the mixture by using thermometer until all sodium hydroxide has dissolved and increase in temperature has stopped.
10.Read the highest temperature reached and record it in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental measurements.
|
|
|
|
Beaker-1 |
|
|
Beaker-2 |
|
Beaker-3 |
|
|
|
|
|
|
|
|
|
|
Mass of beaker (mBeaker) |
|
|
|
................................ |
g |
|
................................ g |
|
................................ g |
|
|
|
|
|
|
|
|
|
|
Mass of sodium hydroxide (m |
NaOH |
|
|
|
g |
|
- |
|
g |
|
|
|
................................ |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
Initial temperature ( i |
|
|
|
................................ |
oC |
|
................................ oC |
|
................................ oC |
|
|
|
|
|
|
|
|
|
|
Highest temperature ( f |
|
|
|
................................ |
oC |
|
................................ oC |
|
................................ oC |
|
|
|
|
|
|
|
|
|
|
Temperature difference ( |
|
|
|
................................ |
oC |
|
................................ oC |
|
................................ oC |
Experiment – 44 Heat of reaction and Hess’s law |
115 |
|

CALCULATIONS
1.Calculate the released energy by dissolution of sodium hydroxide in water in the beaker 1.
QWater = mWater x cWater x T = 100 (g) x 4.184 (J/g°C) x .......... |
(°C) = |
........... J = |
............ kJ |
|
||
QBeaker = mBeaker x cGlass x T = |
........... |
(g) x 0.84 (J/g°C) x .............. |
|
(°C) = ................ |
J = ................. |
kJ |
QR1 = QWater + QBeaker = ................. |
+ |
................ = .............. |
kJ |
|
|
|
2.Calculate the released energy by neutralisation of sodium hydroxide solution with hydrochloric acid in the beaker 2.
QWater = mWater x cWater x T = 100 (g) x 4.184 (J/g°C) x .......... |
(°C) = |
........... J = |
............ kJ |
|
||
QBeaker = mBeaker x cGlass x T = |
........... |
(g) x 0.84 (J/g°C) x .............. |
|
(°C) = ................ |
J = ................. |
kJ |
QR2 = QWater + QBeaker = ................. |
+ |
................ = .............. |
kJ |
|
|
|
3.Calculate the released energy by neutralisation of sodium hydroxide pellets with hydrochloric acid in the beaker 3.
QWater = mWater x cWater x T = 100 (g) x 4.184 (J/g°C) x .......... |
(°C) = |
........... J = |
............ kJ |
|
||
QBeaker = mBeaker x cGlass x T = |
........... |
(g) x 0.84 (J/g°C) x .............. |
|
(°C) = ................ |
J = ................. |
kJ |
QR3 = QWater + QBeaker = ................. |
+ |
................ = .............. |
kJ |
|
|
|
4.Calculate the heat of dissolution of 1 mol sodium hydroxide in water in beaker 1.
H1 |
QR1 |
.................. kJ |
kJ/mol |
= ————— x MWNaOH |
= ———————— x 40 g/mol = ................. |
||
|
mNAOH |
.................. g |
|
5.Calculate the heat of neutralisation of 1 mol sodium hydroxide with hydrochloric acid solution in beaker 2.
H2 |
QR2 |
QR2 |
.................. kJ |
kJ |
= ————— x 1 mol = ———————— = ———————— x 1 mol = ................. |
||||
|
MNaOH |
MNAOH x VNaOH |
1 x 0,05 |
|
6.Calculate the heat of the reaction of one mole sodium hydroxide pellets and hydrochloric acid solution in beaker 3.
H |
|
= |
QR3 |
x MW |
|
= |
.................. kJ |
x 40 g/mol = ............... |
kJ |
3 |
NaOH |
|
|||||||
|
|
||||||||
|
|
mNaOH |
|
g |
|
|
|||
|
|
|
|
|
|
|
|||
7.Calculate the addition of H1and H2.
H4= H1 + H2 = ............... |
+ ............... |
= ............... |
kJ |
Experiment – 44 Heat of reaction and Hess’s law
116

EVALUATIONS AND CONCLUSIONS 


1.Do the experimental results prove the Hess’s law? Explain by Comparing the H3 and H4 since they should be equal.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Express the released energy as heat of the reaction for each reaction in the beakers.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Apply the Hess law and write the reaction equations for the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What are the some possible sources of error in this experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 44 Heat of reaction and Hess’s law |
117 |
|
How can the rate of a reaction be measured?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the rate of the decomposition of hydrogen peroxide.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Separatory funnel |
(1) |
• Delivery glass tube |
(1) |
• Hydrogen peroxide, %20 |
• Erlenmeyer flask, 250 mL |
(1) |
• Rubber tubing, 30 cm |
(1) |
• Manganese(IV) oxide |
• Beaker, 500 mL |
(1) |
• Rubber stopper, with 2 holes |
(1) |
• Distilled water |
• Gas measuring tube, 50 mL |
(1) |
• Support base |
(2) |
|
• Graduated cylinder, 50 mL |
(1) |
• Support rod |
(2) |
|
• Graduated cylinder, 10 mL |
(1) |
• Bosshead |
(2) |
|
• Right-angled glass tube, short |
(1) |
• Universal clamp |
(1) |
|
PRE-LAB DISCUSSION
The rate of a chemical reaction simply refers to the amount of reaction which occurs in unit time, and therefore, if we wish to measure the rate of a chemical reaction we must choose some property of the reaction which will indicate how far the reaction has changed, and observe the way in which the magnitude of that property varies with time.
Thus, for a reaction in which a gas is liberated, it may be possible to collect the gas and measure the way in which the volume of the gas increases with time, in order to obtain the rate of reaction.
PROCEDURE
Set-up
—Put half spatula of manganese(IV) oxide into the erlenmeyer flask.
—Insert the separatory funnel into one of the rubber stopper’s holes and the right-angled glass tube into the other hole.
Note: Lubricate glass-rubber connections with glycerol before connection and twist them in cautiously without force.
—Fill 2/3 of the 500 mL beaker with tap water.
—Connect the long arm of the right-angled glass tube with the delivery tube by using rubber tubing.
—Assemble the apparatus as seen in the Figure.
Note: Be sure that all stoppers and connections are tight.
—Close the stopcock of the separatory funnel. Measure 48 mL of distilled water and 2 mL of hydrogen peroxide, then pour them in the separatory funnel.
Procedure
1.Open the stopcock of the separatory funnel and allow the mixture goes into the erlenmeyer flask.
2.Start the chronometer and measure the volume of the collected gas in the gas measuring tube in a minute for 5 minutes. Record the volumes in the Table in “Observations and Data Tables”.
Note : Close the stopcock of the separatory funnel when all of the mixture has gone into the flask.
Experiment – 45 How can the rate of a reaction be measured?
118
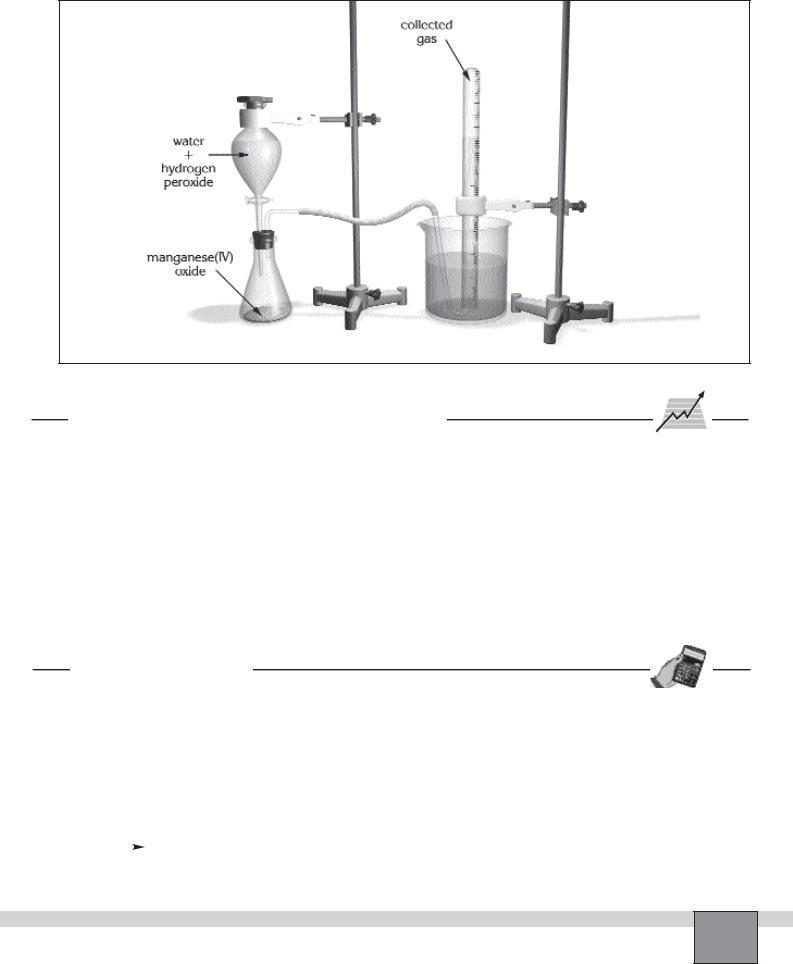
Figure
OBSERVATIONS AND DATA TABLES
1.Note your observations in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with the volume of gas in 1st, 2nd, 3rd, 4th and 5th minutes.
Minute |
|
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
|
|
|
|
|
|
|
|
|
|
Volume of gas |
|
mL |
|
mL |
|
mL |
|
mL |
|
mL |
|
|
|
|
|
|
CALCULATIONS
1.Calculate the rate of production of oxygen gas in mL/min.
|
Volume of gas .................. |
mL |
Rate(O |
) = ————————— = ————————— = mL/min |
|
2 |
Time interval |
min |
|
||
2.Find the relation between the rate of decomposition of hydrogen peroxide and the rate of production of oxygen gas by using balanced reaction equation, then calculate the rate of decomposition of hydrogen peroxide in mL/min.
H2O2 |
|
H2O + 1/2O2 |
|
|
|
|
|
|
|
||
Rate(H2O2) = |
.................... x Rate(O2) = ................. |
x ................ |
= .............. |
mL/min |
|
Experiment – 45 How can the rate of a reaction be measured? |
119 |
|
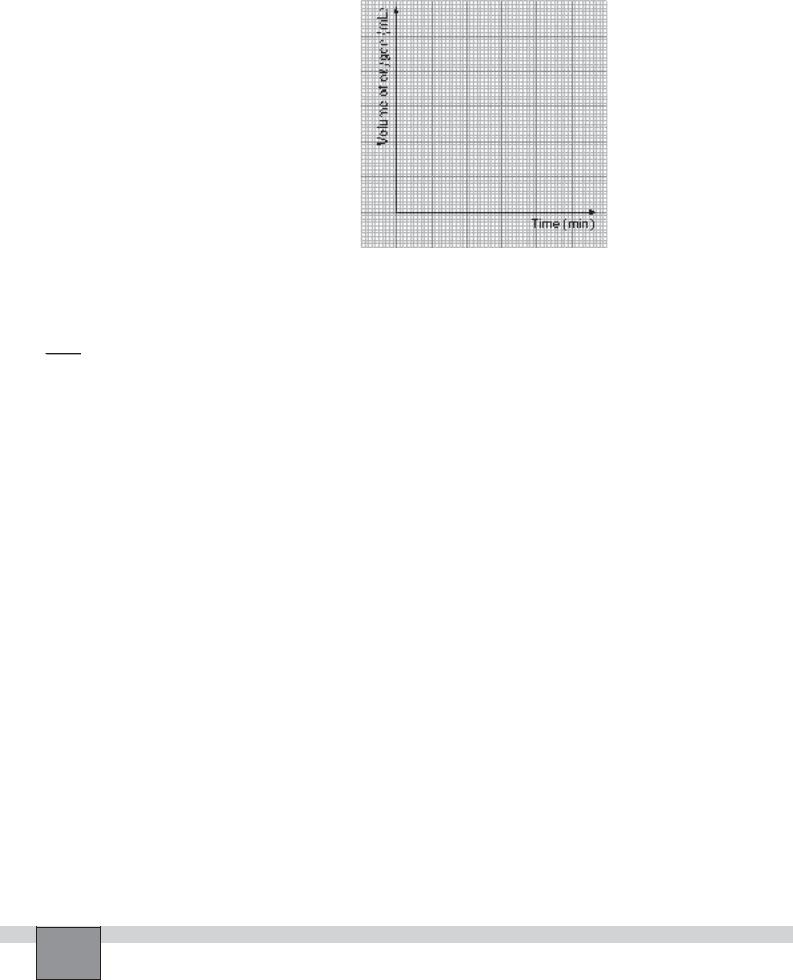
3.Plot the volume of oxygen gas against time.
EVALUATIONS AND CONCLUSIONS 


1.Interpret the graph in the calculation part. What does it tell us?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Compare the rate of the production of oxygen at the beginning and at the end of the experiment. Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Can instant rate be determined by using graph? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Explain which other methods can be used to determine the rate of a reactions.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 45 How can the rate of a reaction be measured?
120
Does the rate of a reaction depend on the interacting surface area?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe the effect of the interacting surface area on the reaction rate.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Test tube |
(2) |
• Hydrochloric acid, 6M |
• Graduated cylinder, 10 mL |
(1) |
• Chalk, powdered |
• Test tube rack |
(1) |
• A Lump chalk (or marble) |
• Protective glasses |
(1) |
• Distilled water |
PRE-LAB DISCUSSION
On the basis of elementary kinetic theory a chemical reaction will occur only if the particles of reacting substances collide each other. The rate of the reaction would therefore be expected to depend on the number of collisions with time. In order to increase the number of collision in a unite time, interacting surface area of the reactants must be in-
creased. When a reaction is a heterogeneous in which reactants are in different phase the particle size of the reactants may influence the rate of the reaction more.
Thus in such reactions, the particle size of the substances should be decreased to increase reaction rate.
PROCEDURE
Set-up
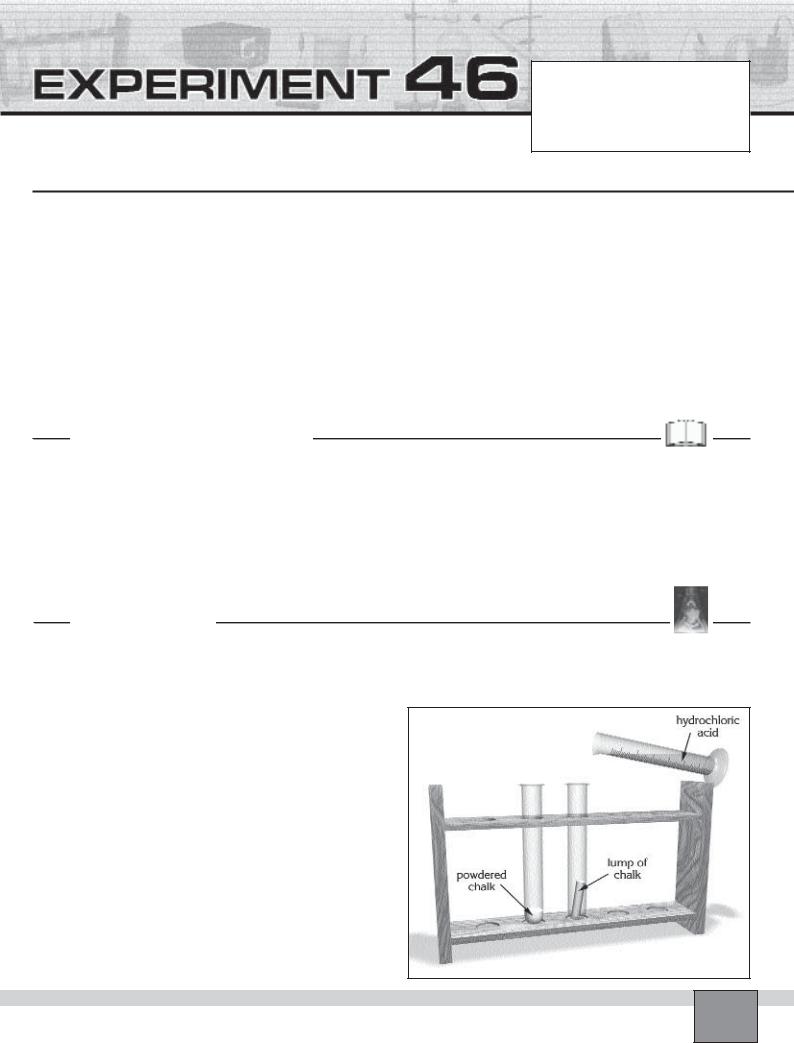
—Place two test tubes in the test tube rack and label them as 1 and 2.
—Put about 0.5 cm depth of powdered chalk in the test tube 1 and a lump of chalk in the test tube 2.
Note: Using a lump of marble instead of chalk decreases the reaction rate more. Marble and chalk consist of the same chemical substance mainly.
— Add 3 mL depth of distilled water in each test tube with graduated cylinder.
—Place 2 mL 6M hydrochloric acid in the graduated cylinder.
Caution: Since acids are corrosive , handle them with great care.
— Wear protective glasses
Procedure
1.Take the test tube 1 and shake it to get suspension of powdered chalk.
2.Hold the test tube 1 with your left hand and graduated cylinder with your right hand over the sink.
Figure
Experiment – 46 Does the rate of a reaction depend on the interacting surface area? |
121 |
|

3.Pour the 6M hydrochloric acid into the test tube 1 and record your observations in “Observations and Data Tables”.
Caution: Since the reaction is vigorous, acid solution may overflow onto your hands. In such cases, rinse your hand with water.
4.Place 2 mL 6M hydrochloric acid in the graduated cylinder.
5.Pour the 6M hydrochloric acid into the test tube 2 and record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations on powdered chalk.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on the lump of chalk.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.How does the interacting surface area effects the reaction rate? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How can the interacting surface area of substances be increased? Explain with examples.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Does the increase in interacting surface area make the impossible reaction possible? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 46 Does the rate of a reaction depend on the interacting surface area?
122
