
experment book
.pdf
3.Stop heating and investigate the products as follows.
4.Take the apparatus apart and clamp the test tube containing collected liquid over the burner. Clamp the thermometer so that its bulb is in the air space above the liquid. Heat the liquid gently until boiling point. determine the boiling point of the liquid. Record the result in
Figure-A
the “Observations and Data Tables”.
5.Transfer some of the residual salt into the watch glass, then add some of the cold collected liquid on the salt. Note your observations in the “Observations and Data Tables”.
Figure-B
Part-B
Set-up
—Finely powder the copper(II) crystals in the mortar.
—Set up the apparatus as seen the Figure.
Procedure
1.Weigh the evaporating dish. Record the result in the Table in part-B in “Observations and Data Tables”.
2.Place a spatula of finely ground copper(II) sulphate in the evaporating dish. Then re-weigh the evaporating dish with salt. Record the results in the Table in part-B “Observations and Data Tables”.
3.Heat the evaporating dish until no further change takes place. Note your observations in the “Observations and Data Tables” in part-B.
Note: It requires about 5 minutes.
4.Stop heating and let the evaporating dish to cool.
5.Re-weigh the evaporating dish. Repeat the heating for a few minutes, cooling and re-weighing until the last two weights are the same. Record the last result in the Table in part-B “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


Part-A
1.Note your observations, What happens when the salt is heated?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2. Boiling point of the collected liquid : ............. |
oC |
3.Note your observation, What happens when the liquid is added on the residual salt?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 24 What is lost when copper(II) sulphate crystals are heated? |
63 |
|
Part-B
1.Note your observations on heating the evaaporating dish.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with your measurements.
Mass of empty evaporating dish |
|
Mass of evaporating dish with salt |
|
Mass of evaporating dish with salt af- |
(W1) |
|
before heating (W2) |
|
ter heating (W3) |
|
|
|
|
|
................. g |
|
................. g |
|
................. g |
EVALUATIONS AND CONCLUSIONS
Part-A
1.Compare its boiling point with that of water. Can it be water? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Is the escaping of water from the salt a chemical change or not? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Part-B
1.Calculate the masses of the salt before and after heating and mass of water below.
Mass of the salt before heating (W4) = W2 - W1 = |
............ - |
............ = |
............ g |
|
Mass of the salt after heating (W5) = W3 - W1 = ............ |
- ............ |
= ............ |
g |
|
Mass of water (W6) = W4 - W5 = ............ |
- ............ ............ |
= |
g |
|
2.Find the mass ratio of the copper(II) sulphate and water as below.
mCopper(II) sulphate / mWater = W5 / W6 = ............ |
/ ............ |
= ............ |
/ ............ |
3.Write the molecular formula of the hydrated copper(II) sulphate by using the above mCopper(II) sulphate / mWater ratio.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Compare your results with your friends’ and theoretical values. What can be the reasons for the differences?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 24 What is lost when copper(II) sulphate crystals are heated?
64
How can chemical and physical changes be distinguished?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To observe chemical reactions and distinguish chemical and physical changes.
EQUIPMENT and MATERIALS:
Part-A
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(2) |
• Burner |
(1) |
• Naphthalene |
• Test tubes rack |
(1) |
• Protective glasses |
(1) |
• Sugar |
• Test tube holder |
(1) |
• Marker pen |
(1) |
• Cotton (or glass wool) |
• Spatula |
(1) |
|
|
• Distilled water |
|
|
|
|
|
Part-B |
|
|
|
|
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(2) |
• Spatula |
(1) |
• Hydrochloric acid, 6M |
• Test tube rack |
(1) |
• Marker pen |
(1) |
• Sodium bicarbonate |
|
|
|
|
• Distilled water |
PRE-LAB DISCUSSION
The changes of matter are classified as either physical or chemical. In physical change, composition of matter remains constant. For example, breaking glass, cutting wood, melting of ice, and evaporation of liquid are physical changes.
A chemical change results in the formation of one or more new substances. These new substances differ in chemical properties and composition from the original substances. For example, rusting of iron, burning of paper are chemical changes.
Energy needed for chemical changes is generally bigger than energy needed for physical changes. Chemical changes occurs in the outermost electrons of atoms. The changes in nucleus of an atom are called nuclear changes. Chemical change is called as chemical reaction. Chemical reaction is shown by a chemical equation using chemical symbols. Such as
Fe(s) + S(s) heat FeS(s)
Chemicals on the left hand side of the equation are the reactants and on the right hand side are the products.
PROCEDURE
Part-A
Set-up
—Place two test tubes in the test tube rack and number them as 1 and 2.
—Place a spatula of naphthalene into the test tube 1, and a spatula of sugar into the test tube 2. Close the opening ends of the test tubes with pieces of cotton (or glass wool).
—Wear protective glasses
Procedure
1.Pick up the test tube 1 with holder and heat the lower part of it with burner gently as seen in the Figure A. Ob-
serve the process taking place and note your observations in the “Observations and Data Tables”.
Caution: Since naphthalene is flammable, heat cautiously.
2.Stop heating and allow the test tube to cool, then replace it in the test tube rack.
3.Repeat the steps 1 and 2 for the test tube 2.
4.Examine the test tube 1 and 2 during and after the cooling process carefully. Record your observations in the “Observations and Data Tables”.
Note: Since naphthalene produces unpleasant and noxious vapours, air the room after the experiment.
Experiment – 25 How can chemical and physical changes be distinguished? |
65 |
|

Part-B
Set-up
—Place two test tubes in the test tube rack and label them as 1 and 2.
—Fill the test tube 1 to a height of 2 cm with distilled water, and fill the test tube 2 to a height of 2 cm with 6 M hydrochloric acid solution.
—Wear protective glasses
Procedure
1.Place a spatula of sodium bicarbonate (NaHCO3) in the test tube 1. Record your observations in the “Observations and Data Tables”.
2.Place a spatula of sodium bicarbonate (NaHCO3) in the test tube 2. Record your observations in the “Observations and Data Tables”.
Figure-A |
Figure-B |
|
|
|
|
OBSERVATIONS AND DATA TABLES 


Part-A
1.Note your observations on naphthalene
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on sugar
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Part-B
1.Note your observations on the sodium bicarbonate in the water
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on the sodium bicarbonate in hydrochloric acid
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 25 How can chemical and physical changes be distinguished?
66

EVALUATIONS AND CONCLUSIONS 


1.What is the fundamental difference between the processes that take place in the test tube 1 and 2?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What process occurred in the test tubes? Represent them by symbols.
Test tube 1 in part-A: .....................................................................................................................................................
Test tube 2 in Part-A: .....................................................................................................................................................
Test tube 1 in part-B: .....................................................................................................................................................
Test tube 2 in Part-B: .....................................................................................................................................................
3.How could one distinguish a chemical change and a physical change? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 25 How can chemical and physical changes be distinguished? |
67 |
|
|
Date |
...............................................................: |
|
|
Partners : ............................................................... |
|
|
|
|
............................................................... |
|
|
|
|
|
Can chemical reactions be classified? |
Grade |
: ............................................................... |
|
|
|
|
|
|
|
|
|
PURPOSE : To observe some chemical reactions and identify reactants and products.
To classify the reactions and write balanced reaction equations.
EQUIPMENT and MATERIALS:
Part-A
Equipment |
|
|
|
Chemicals and Other Materials |
• Crystallising dish |
(1) |
• Universal clamp |
(3) |
• Potassium chlorate |
• Test tube |
(3) |
• Bosshead |
(3) |
• Manganese(IV) oxide |
• Right-angled glass tube |
(1) |
• Balance |
(1) |
• Wood splint |
• Delivery glass tube |
(1) |
• Burner |
(1) |
|
• Rubber tubing, 10 cm |
(1) |
• Rubber stopper with a hole |
(1) |
|
• Support base |
(2) |
• Spatula |
(1) |
|
• Support rod |
(2) |
• Protective glasses |
(1) |
|
|
|
|
|
|
Part-B |
|
|
|
|
Equipment |
|
Chemicals and Other Materials |
|
|
• Crucible tongs |
(1) |
• Copper |
|
|
• Burner |
|
• Emery paper |
|
|
|
|
|
|
|
Part-C |
|
|
|
|
Equipment |
|
Chemicals and Other Materials |
|
|
• Test tube |
(2) |
• Hydrochloric acid, 6M |
|
|
• Test tube rack |
(1) |
• Zinc, granulated |
|
|
• Protective glasses |
(1) |
|
|
|
|
|
|
|
|
Part-D |
|
|
|
|
Equipment |
|
Chemicals and Other Materials |
|
|
• Test tube |
(2) |
• Lead(II) nitrate solution, 0.5 M |
|
|
• Test tube rack |
(1) |
• Potassium iodide solution, 1 M |
|
|
PRE-LAB DISCUSSION
There are many kinds of chemical reactions and several ways to classify them. One useful method classifies reactions into four major types. These are; 1. decomposition, or analysis, 2. combination or synthesis, 3. single replacement, and 4. double replacement.
In a combination reaction, two or more substances combine to form a more complex substance. Equations for synthesis reactions have the general form as A + B AB.
Decomposition reactions are the opposite of the synthesis reaction. That is, in a decomposition reaction a substance
decompose two or more simpler substances.
In a single displacement reaction, one element in a compound is replaced by another which is more active. Equation for single displacement reaction is, X + YB XB + Y, where one element replaces another element
In a double displacement reaction, the metal ions of two different ionic compounds replace with one another. General equations for this type of reaction is as below.
AB+ CD AD + CB
Experiment – 26 Can Chemical reactions be classified?
68
PROCEDURE
Part-A : ANALYSIS REACTION
Set-up
—Place two third of beaker with water. Then fill two test tubes with water and invert them into the water in the crystallising dish.
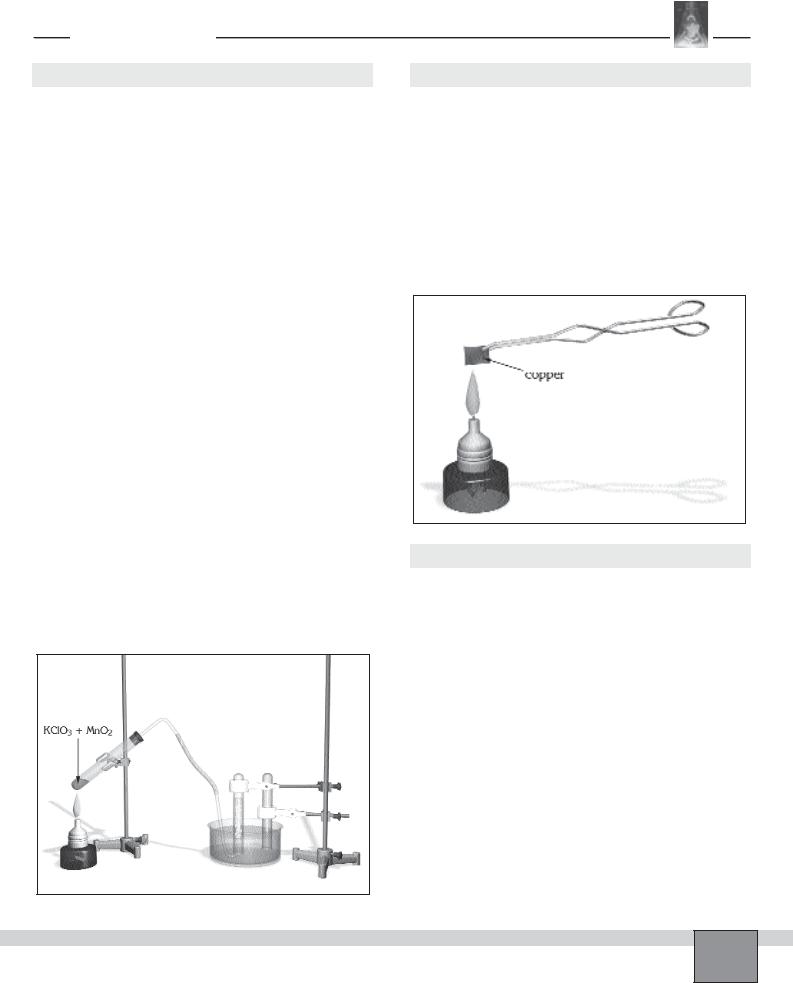
—Place 2 g of potassium chlorate and small amount of manganese(IV) oxide in an another test tube. Then mix them in the test tube with stirring rod. Set the apparatus as seen in the Figure.
Note: Lubricate the glass-rubber connections with glycerol.
— Wear protective glasses
Procedure
1.Heat potassium chlorate gently with a low non-lumi- nous burner flame. Then heat vigorously. Observe the change taking place in the test tube. Record your observations in “Observations and Data Tables”.
2.When the first gas collecting tube is full of gas, shift the lower end of the delivery tube to the other inverted test tube.
Note: Do not take the test tube out from the water.
3.When no more gas is evolved, stop heating.
Note: The delivery tube should be removed from water before heating is stopped. Otherwise water may be forced into the hot test tube.
4.Do smouldering test (oxygen test) for the collected gas in the test tubes. Record your observations in “Observations and Data Tables”.
Smouldering test: Close the opening of the tube with your thumb and take the test tube out. Ignite a splint. Then blow the flame out. Immediately, remove your thumb and Insert the smouldering end of the splint into the tube.
Figure
Part-B : SYNTHESIS REACTION
Set-up
—Clean the surface of a piece of copper metal with emery paper until the metal is shiny.
Procedure
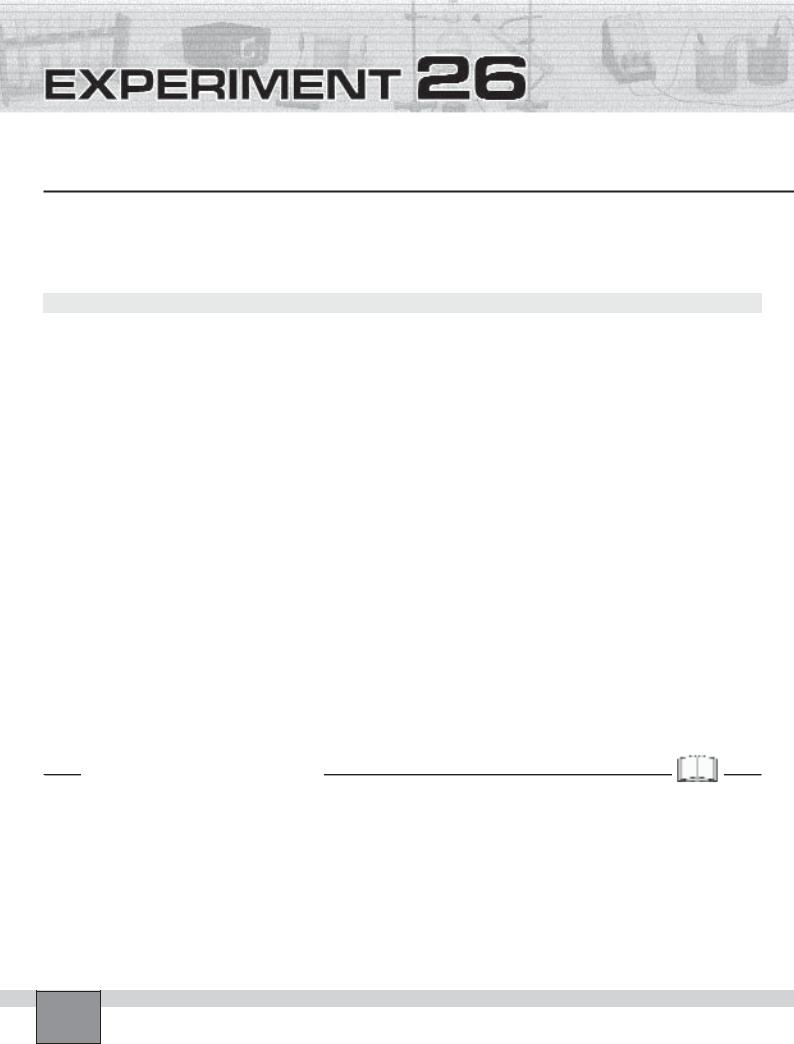
1.Hold the copper metal with crucible tongs in the hottest part of a the burner flame as in the Figure for 1- 2 minutes.
2.Examine the appearance of copper metal and note your observations in “Observations and Data Tables”.
Figure
Part-C : SINGLE DISPLACEMENT REACTION
Set-up
—Place two clean test tubes in the test tube rack. Fill one of the test tubes to a height of 2 cm with 6 M hydrochloric acid.
—Wear protective glasses.
Caution: Handle the acid solutions carefully. Do not inhale any HCl fumes.
Procedure
1.Carefully put a piece of zinc metal into the hydrochloric acid. Record your observations in “Observations and Data Tables”.
2.Put the empty test tube with the opening end down over the test tube as seen in the Figure-1.
3.After about 1 minute, carry out hydrogen test for the gas which is collected in the inverted test tube. Record your observations in “Observations and Data Tables”.
Hydrogen test : Close the opening of the tube with your thumb. Move the test tube toward the flame, remove your thumb and hold the opening of the tube close to the flame as in the Figure-2.
Experiment – 26 Can Chemical reactions be classified? |
69 |
|

Figure-1 |
Figure-2 |
|
|
|
|
|
|
|
Part-D : DOUBLE DISPLACEMENT REACTION
Set-up
—Place two test tubes in the test tube rack and label them as 1and 2 with marker pan.
—Fill the test tube 1 with lead(II) nitrate solution to a height of 3 cm, and the test tube 2 with sodium iodide solution to a height of 2 cm .
Procedure
1.Take the test tube 2 and pour the solution into the test tube 1.
2.Record your observations in “Observations and Data Tables”.
Note: The experiment can be carried out with silver nitrate and sodium chloride solutions.
Figure
Experiment – 26 Can Chemical reactions be classified?
70

OBSERVATIONS AND DATA TABLES 


Part-A
1.Note your observations on heating the potassium chlorate.
...........................................................................................................................................................................................
2.Note your observations about smouldering test.
...........................................................................................................................................................................................
Part-B
1.Note your observations on the copper.
...........................................................................................................................................................................................
Part-C
1.Note your observations on zinc in hydrochloric acid.
...........................................................................................................................................................................................
2.Note your observations about hydrogen test.
...........................................................................................................................................................................................
Part-D
1.Note your observations after mixing of the solutions .
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


Part-A
1.What is the characteristic property of the analysis reactions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What are the substance that produced in the experiment? Write the balanced reaction equation.
...........................................................................................................................................................................................
Part-B
1.What is the characteristic property of the synthesis reactions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the substance that produced in the experiment? Write the balanced reaction equation.
...........................................................................................................................................................................................
Part-C
1.What is the characteristic property of the single-displacement reactions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the balanced reaction equation for the experiment.
...........................................................................................................................................................................................
Part-D
1.What is the characteristic property of the double-displacement reactions? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the substance that precipitates in the experiment? Write the reaction equation.
...........................................................................................................................................................................................
Experiment – 26 Can Chemical reactions be classified? |
71 |
|

How can hydrogen gas be produced in laboratory?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare hydrogen gas in laboratory and to investigate its properties.
EQUIPMENT and MATERIALS:
Equipment |
|
• Rubber tubing, 10 cm |
(1) |
Chemicals and Other Materials |
• Crystallising dish |
(1) |
• Rubber stopper, with a hole (1) |
• Sulphuric acids, 1 M |
|
• Beaker, 250 mL |
(1) |
• Support base |
(2) |
• Zinc granulated |
• Test tube |
(3) |
• Support rod |
(2) |
• Copper sulphate solution 0,5 M |
• Dropper |
(1) |
• Universal clamp |
(3) |
• Soap solution |
• Right - angled glass tube |
(1) |
• Bosshead |
(3) |
|
• Delivery tube, with tip |
(1) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Hydrogen is unique among the elements. It does not really belong to any particular group in the periodic table. In some versions of the table, hydrogen is separated from the main body of the elements. Although hydrogen is classified as a nonmetal, it combines with almost all elements. The hydrogen atom consists of a single proton and one electron that
is situated very close to the nucleus. It forms covalent compounds with other nonmetals by sharing its electron. In such compounds, the oxidation state of hydrogen is +1. Hydrogen also combines with some metals in group 1 and group 2 to form ionic compounds, called metal hydrides, in which it has an oxidation state of - 1.
PROCEDURE
Set-up
—Fill the crystallising dish about 2/3 full of distilled water.
—Fill the 250 mL beaker about 2/3 full of detergent solution.
—Fill one of the test tubes (or graduated cylinder) with water. Close the mouth of the tube with your thumb (or a piece of paper), then sink into the water in the beaker. Open the mouth of the tube. Apply the same process for a second test tube.
Note: Lubricate glass-rubber connections with glycerol before connection.
— Wear protective glasses.
Procedure
1. Fill another test tube with 3 cm height of 1M sulphuric acid. Add a few drops of copper sulphate solution.
— Wear protective glasses.
Note: Addition of copper sulphate speeds up the reaction.
2. Place a few pieces of granulated zinc into the test tube
which contains acid solution, then immediately assemble the apparatus.
3.Collect the hydrogen gas over water in the inverted tube. When the first gas collecting tube is full of gas, shift the lower end of the delivery tube to the other inverted test tube.
4.Take the delivery tube out from the water and sink into the detergent solution. Blow some soap bubbles by produced hydrogen gas. Record your observations in “Observations and Data Tables”.
5.Do hydrogen test for the collected gas in the test tubes. Record your observations in “Observations and Data Tables”.
Note: First test tube may contain mainly displaced air.
Hydrogen test: Close the opening of the tube with your thumb. Move the test tube toward the flame, remove your thumb and place the opening the tube close the flame.
Caution: Never light the hydrogen gas as it comes out of the delivery tube and never heat the acid solution.
Experiment – 27 How can hydrogen gas be produced in laboratory?
72
