
experment book
.pdf
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of matter is used for the separation of the pepper and table salt mixture?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Which other matter can be separated by the method used in the experiment? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Is the pepper and table salt mixture homogeneous or heterogeneous mixture? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.What are the characteristic properties of the pepper and table salt observed in the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Can you suggest another method to separate pepper and table salt mixtures?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 10 Can an electrified object be used to separate mixtures? |
33 |
|
How can salt be recovered from a mixture of salt and sand?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate salt and sand mixture and to obtain them individually.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 150 mL |
(2) |
• Sand |
• Erlenmeyer flask, 200 mL |
(1) |
• Table salt |
• Funnel |
(1) |
• Distilled water |
• Dropper |
(1) |
• Filter paper |
• Stirring rod |
(1) |
|
• Spatula |
(1) |
|
• Evaporating dish |
(1) |
|
• Tripod |
(1) |
|
• Wire gauze |
(1) |
|
• Burner |
(1) |
|
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Solubility of substances in water can be used to separate mixtures. Insoluble substances do not dissolve and retain on the filter paper after filtration. Soluble substances can be ob-
tained by evaporating water of the mixture. This method is used in industry widely.
PROCEDURE
Set-up
—Take a spatula-tip full of table salt and a spatula-tip full of pre-washed sand on a piece of paper, then mix them.
—Fill the half of the 150 mL beaker with distilled water then put the mixture into the beaker.
—Stir the mixture until the salt dissolves.
Note: The sand does not dissolve in water.
—Place the folded filter paper into funnel. Place the funnel on the erlenmeyer flask as seen in the Figure-2.
Note: Moisten the folded filter paper slightly and press it firmly against the side walls of the funnel so that it fits closely.
— Wear protective glasses.
Procedure
1.Pour the mixture in the beaker through the filter paper.
Note: When the all mixture is poured, rinse the beaker with some more water and pour the extra water through the funnel.
2.Take the sand retained on the filter paper on a piece of paper.
3.Place about 10 drops of residual solution in the erlenmeyer flask into a evaporating dish with a dropper.
4.Place the evaporating dish on the middle of the wire gauze, then heat it cautiously with the smallest burner flame. Observe the re-crystallisation of the salt.
Caution: Do not heat the evaporating dish vigorously, the salt may spread.
5.Extinguish the burner flame when the water has nearly completely evaporated, then let the evaporating dish to cool. Examine the residue and record your observations in “Observations and Data tables”.
Experiment – 11 How can salt be recovered from a mixture of salt and sand?
34

Figure-1 |
|
Figure-2 |
|
Figure-3 |
|
|
|
|
|
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which properties of the sand and the table salt are used to separate the mixture in the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How table salt can be industrially obtained from rock salt and sea water? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Classify the mixtures in the experiments as homogeneous or heterogeneous?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.According to experimental result, what are the characteristic properties of the sand and table salt?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Propose a method to separate the mixture which is composed of iron filings, sulphur powder, table salt, sand and water.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 11 How can salt be recovered from a mixture of salt and sand? |
35 |
|
How can two immiscible liquids be separated?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate immiscible olive oil and water liquids.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Beaker, 150 mL |
(1) |
• Support rod |
(1) |
• Olive oil |
• Separatory funnel |
(1) |
• Bosshead |
(1) |
|
• Graduated cylinder, 50 mL |
(1) |
• Universal clamp |
(1) |
|
• Support base |
(1) |
|
|
|
PRE-LAB DISCUSSION
When liquids do not mix, they form layers. Liquids are arranged in order according to their density. A liquid which
is the most denser stays at the lower layer. The liquids are separated individually layer by layer.
PROCEDURE
Set-up
Figure
—Place 150 mL beaker on the bench, then measure 25 mL of water with a 50 mL graduated cylinder and pour it into the beaker.
—Add 15 mL of olive oil into the beaker. Stir the mixture with a stirring rod.
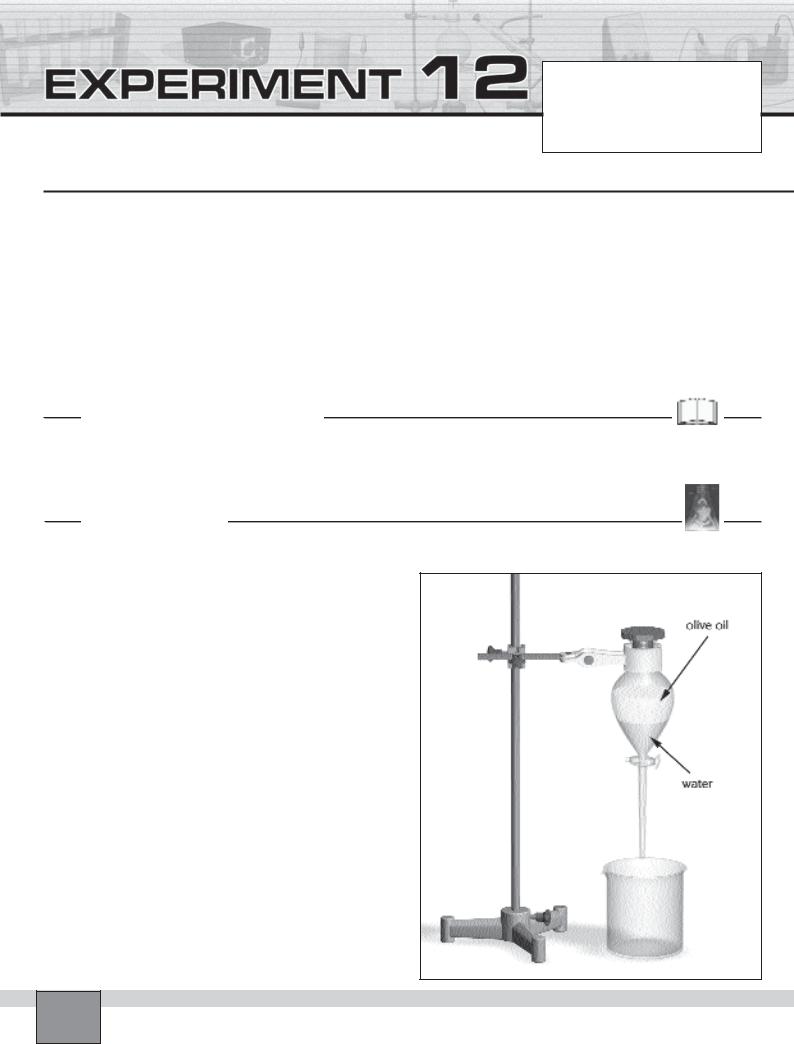
—Close the stopcock of the separatory funnel (horizontal position), then pour the mixture into the separatory funnel. Close the separatory funnel with its stopper and shake it vigorously.
Note: Hold the separatory funnel’s stopper down with your thumb)
—Fix the separatory funnel on the support rod and place the beaker as seen in the Figure.
—Wait for 2-3 minutes and record your observations in “Observations and Data tables”.
Procedure
1.Remove the stopper of the separatory funnel.
2.Open the stopcock of the separatory funnel slowly to let the lower liquid layer to run out.
3.Close the stopcock when the liquid boundary has been reached.
4.Examine the separated liquids and record your observation in “Observations and Data tables”.
Experiment – 12 How can two immiscible liquids be separated?
36

OBSERVATIONS AND DATA TABLES 


1.Note your observations on the liquids in the beaker and the separatory funnel.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which properties of matter allow the water and olive oil to be separated?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How this method is used in industry? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.According to the experimental results, which characteristic properties of the liquids are observed?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 12 How can two immiscible liquids be separated? |
37 |
|

Can a mixture of miscible liquids be separated? (Distillation)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate water and ethyl alcohol by distillation.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Distillation flask |
(1) |
• Tripod |
(1) |
• Distilled water |
• Condenser |
(1) |
• Wire gauze |
(1) |
• Ethyl alcohol |
• Beaker, 150 mL |
(1) |
• Burner |
(1) |
• Pieces of porcelain |
• Erlenmeyer flask,100 mL |
(1) |
• Rubber stopper with a hole |
(2) |
• Wood splint |
• Thermometer |
(1) |
• Support rod |
(2) |
|
• Graduated cylinder, 50 mL |
(1) |
• Support base |
(2) |
|
• Watch glass |
(2) |
• Bosshead |
(2) |
|
• Dropper |
(1) |
• Universal clamp |
(2) |
|
• Stirring rod |
(1) |
• Rubber tube, 50 cm |
(2) |
|
• Spatula |
(1) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
A mixture which is composed of miscible liquids can be separated into its components by using the boiling point differences of the liquids. When a such mixture is heated, the liq-
uid which has lower boiling point starts boiling first. The distillate contains all other liquids in small ratio. Because liquids evaporates at any temperature.
PROCEDURE
Set-Up
— Set-up the apparatus as shown in the Figure.
Note: The thermometer bulb should be in the line with the sidearm of the distillation flask.
Note: The cooling water should enter through the lower tube of the condenser.
Note: Lubricate glass-rubber connections with glycerol.
—Place 50 mL of distilled water and 30 mL of ethanol in a 150 mL beaker with graduated cylinder. Pour approximately 5 mL of the mixture in a watch glass.
—Pour the rest of the mixture into the distillation flask and add a few pieces of porcelain, then close the flask with rubber stopper
—Wear protective glasses.
Procedure
1.Ignite the burner and heat the mixture. Read the temperature every 2 minutes and record your observation in “Observations and Data tables”.
2.When the temperature begins to rise above 82oC, stop heating.
3.Transfer approximately 5 mL of distillate, which has been collected in the erlenmeyer flask, to the second watch glass.
4.Light the burner and ignite a wood splint. Try to ignite the liquids on the watch glasses with the splint. Record your observations in “Observations and Data tables”.
Experiment – 13 Can a mixture of miscible liquids be separated? (Distillation)
38

Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on burning test of the mixture and of the collected liquid.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of matter allow the water and ethanol to be separated by distillation?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the task fulfiled by the condenser?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.How it can be decided that a mixture is homogeneous or heterogeneous?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 13 Can a mixture of miscible liquids be separated? (Distillation) |
39 |
|
Can gas mixtures be separated?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To separate the gas mixtures formed by decomposition of lead(II) nitrate.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Beaker, 500 mL |
(1) |
• Rubber stopper, with a hole |
(1) |
• Lead (II) nitrate |
• Test tube |
(3) |
• Support rod |
(3) |
• Table salt |
• Spatula |
(1) |
• Support base |
(3) |
• Ice |
• U shaped glass tube |
(1) |
• Bosshead |
(3) |
• Wood splint |
• Glass tube, 10 cm |
(1) |
• Universal clamp |
(3) |
|
• Delivery tube, with tip |
(1) |
• Rubber tubing, 20 cm |
(2) |
|
• Burner |
(1) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Gases can form mixtures in any ratio. Gas mixtures can also be separated its components by physical methods. Boiling point difference is useful to separate gas mixtures. If a gas mixture is passed through a cold medium which is
arranged according to the gas having higher boiling point, the gas condenses and stays in the cold medium. The other gases that have lower boiling point pass through the cold medium.
PROCEDURE
Set-up
—Place a 3 cm depth of finely-powdered lead(II) nitrate in a test tube and fix the tube on the support rod with a clamp at an angle.
—Fill the two third of the 500 mL beaker with ice and table salt.
Note: In alternate layers, use about four parts of crushed ice to one salt for ice bath.
—Fill another test tube with water and close the test tube successively with your thumb or a piece of paper, then sink it upside down in the water in crystallising dish. then clamp it on the support rod.
—Attach the rubber tubing with the both end of the U tube and sink the U tube into the salty ice.
—Attach the right-angled glass tube and rubber stopper
Note: Lubricate glass rubber connections with glycerol always .
—Assemble the apparatus as seen in the Figure.
—Wear protective glasses.
Procedure
1.Ignite the burner and gently heat the upper part of the test tube to make the air goes out. Then stop heating and re-fill the gas collecting tube.
Note: Do not heat the lead(II) nitrate in the tube.
2.Heat the test tube containing lead(II) nitrate, gently at first then vigorously. When the decomposition of the salt finish, stop heating. Note your observations in “Observations and Data Tables”.
3.Observe the condensed gas in the U-tube. Record your observations in “Observations and Data Tables”.
4.Pick up a wood splint and ignite it in the flame. Allow about 0.5 cm to burn off. Then below the flame out.
5.Take the gas collecting tube out and immediately insert the smouldering end of the splint into the test tube. Record your observations in “Observations and Data tables”.
Experiment – 14 Can gas mixtures be separated?
40

Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations on heating the lead(II) nitrate.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on the collected gases.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Which property of gases is used to separate them?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the chemical reaction of the decomposition of the lead (II) nitrate.
...........................................................................................................................................................................................
3.Which gas is collected in the U-tube? Why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Which gas is collected in the gas collecting tube? Why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.How oxygen gas is industrially obtained from air? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
6.Can you suggest an alternative method to separate gas mixtures? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 14 Can gas mixtures be separated? |
41 |
|
Can melting point difference be used to separate metal mixtures?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : |
To separate mixture of metal fillings by heating. |
|
EQUIPMENT and MATERIALS: |
|
|
Equipment |
|
Chemicals and Other Materials |
• Crucible |
(2) |
• Iron filings |
• Clay triangle |
(1) |
• Tin |
• Tong |
(1) |
|
• Tripod |
(1) |
|
• Burner |
(1) |
|
• Balance |
(1) |
|
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Solid mixtures can be separated according to melting points of solids. Metal mixtures are also solid mixtures. When a metal mixture is heated, the metal which has lower melting
point, melts first. This method is used in industry especially in metallurgy.
PROCEDURE
Set-up |
Figure |
|
— Weigh 5 g of iron filings and 5 g of tin. Put them into |
||
|
||
a crucible (or an evaporating dish). |
|
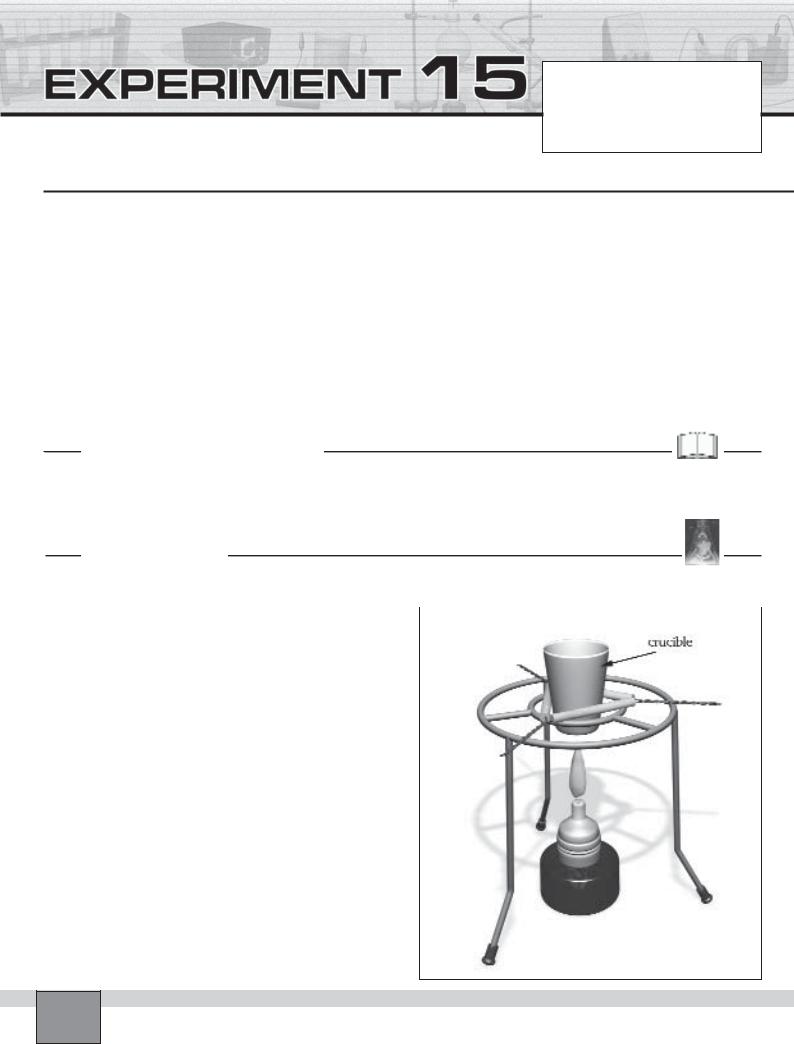
—Place the crucible with clay triangle on the tripod as in the figure.
—Wear protective glasses.
Procedure
1.Ignite the burner and heat the crucible vigorously as seen in the Figure. Record your observations in “Observations and Data Tables”.
2.Extinguish the burner flame, when one of the metal has melt.
3.Hold the crucible with a crucible tong and pour carefully the molten metal into another crucible.
Note: Do not put the hot crucible on cold surface, it may crush.
4.Examine the molten metal and the other metal then record your observations in “Observations and Data Tables”.
Experiment – 15 Can melting point difference be used to separate metal mixtures?
42
