
experment book
.pdf
4. |
Lift up the stopper with thermometer |
Figure |
|
briefly and add two spoonfuls of oxalic acid |
|
|
|
|
|
into the distillation flask. Close the distilla- |
|
|
tion flask with the stopper. |
|
Note: Second addition of oxalic acid is necessary, otherwise the yield of formic acids is very small.
5.Ignite the burner again and heat the mixture so that the temperature reaches 110°C.
6.Stop heating as soon as about 6-7 mL of distillate have been collected in the erlenmeyer flask.
7.Pick up a little condensate with a dropper and put a few drops on the blue litmus paper. Carefully test the smell of the distillate. Record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations you made ;
on heating the oxalic acid-glycerol mixture : ......................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on addition of formic acid on the litmus paper: ................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on testing of smell of formic acid : ....................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw a conclusion from your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the role of glycerol?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the properties of formic acid that you observed in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Write the chemical equations for the preparation of formic acid.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 71 How can formic acid be prepared? |
183 |
|
Does formic acid represent all carboxylic acids?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the properties of formic acid.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
|
|
• Test tube |
(7) |
• Formic acid |
• Ammoniacal silver nitrate solution |
|
• Test tube rack |
(1) |
• Granulated zinc |
• Sodium hydroxide, 1M |
|
• Beaker, 250 mL |
(1) |
|||
• Methyl red solution |
• Sodium hydroxide pellets |
|||
• Dropper |
(1) |
|||
• Protective glasses |
(1) |
• Phenolphthalein solution |
|
PRE-LAB DISCUSSION
Formic acid is the first member of the carboxylic acids. It represents the properties of other carboxylic acids beside its special properties.
PROCEDURE
Set up
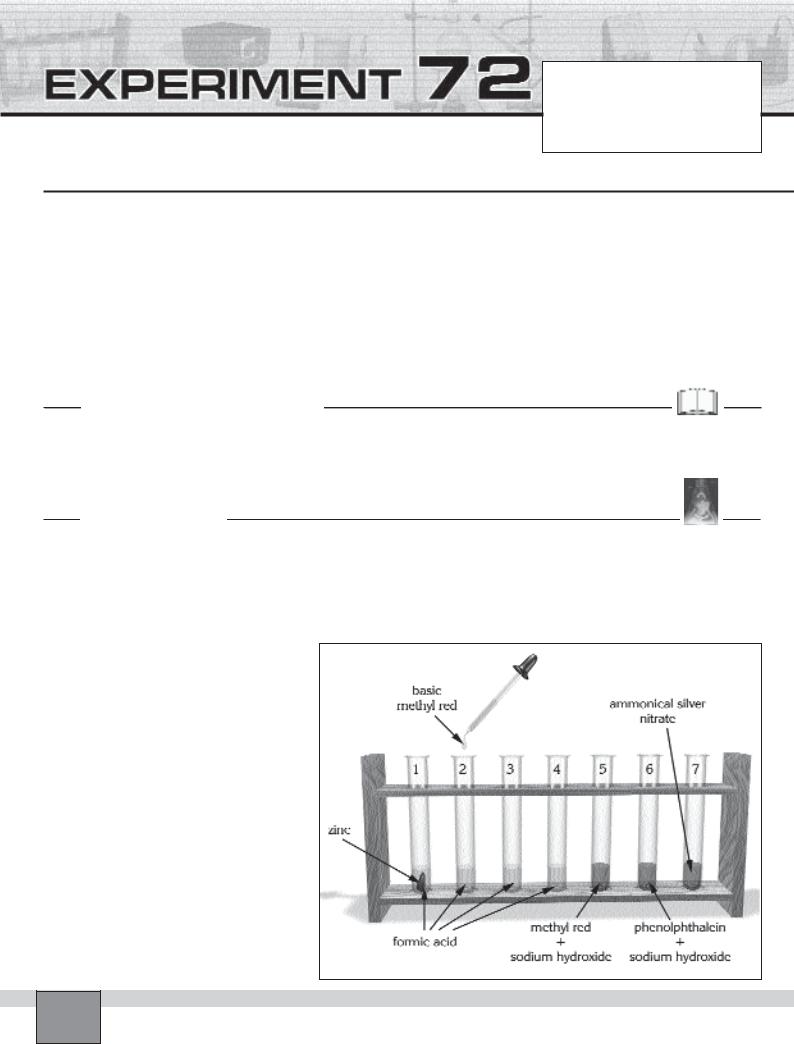
—Place 7 test tubes in the test tube rack and number them from 1 to 7 with marker pen.
—Place about 1 mL of formic acid into test tubes from 1 to 4 individually.
Caution: Do not swallow formic acidic or let it to Figure contact your skin.
—Put a few drops of methyl red into the test tube 5 and add the sodium hydroxide solution until the colour of the methyl red solution becomes yellow.
—Place a few drops of phenolphthalein solution into the test tube 6 and add the sodium hydroxide solution until the colour of the phenolphthalein solution becomes pink.
—Place about 5 mL ammoniacal silver nitrate solution into the test tube 7
—Wear protective glasses.
Procedure
1.Put granulated zinc into the first test tube. Record your observations in “Observations and Data Tables”.
2.Take a few drops of methyl red solution with a dropper in the test tube 5 and add
a few drops of this solution onto formic acid in the the test tube 2. Record your observations in “Observations and Data Tables”.
Note: Clean dropper before using again clearly.
Experiment – 72 Does formic acid represent all carboxylic acids?
184

3.Take a few drops of phenolphthalein solution with a dropper in the test tube 6 then add a few drops of this solution onto formic acid in the the test tube 3. Record your observations in “Observations and Data Tables”
4.Put small piece of a sodium hydroxide pellet onto the ammoniacal silver nitrate solution in test tube 7.
5.Fill the two third of the beaker with hot water then take the test tube 7 from the rack and place it in the water.
6.Take a few drops of formic acid in the test tube 4 with a dropper then add it onto the ammoniacal silver nitrate solution in test tube 7. Record your observations in “Observations and Data Tables”
OBSERVATIONS AND DATA TABLES 


1.Note the observations you made on formic acid with;
Granulated Zinc : .........................................................................................................................................................
...........................................................................................................................................................................................
Methyl Red solution : ..........................................................................................................................................................
...........................................................................................................................................................................................
Phenolphthalein solution : ............................................................................................................................................
...........................................................................................................................................................................................
Ammoniacal silver nitrate solution : ...............................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw a conclusion in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the properties of formic acid that you observed in the experiment. Find non-observed properties of it from your text book.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write chemical equation for the reaction between zinc and formic acid.
...........................................................................................................................................................................................
4.Which properties of formic acid represents the other carboxylic acids? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Why does the formic acid be reduced but the other carboxylic acids do not?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 72 Does formic acid represent all carboxylic acids? |
185 |
|

How can acetic acid be obtained?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare acetic acid in the laboratory with anhydrous sodium acetate and concentrated sulphuric acid.
EQUIPMENT and MATERIALS:
Equipment |
|
• Support rod |
(2) |
• Spatula |
(1) |
• Distillation flask, 500 mL |
(1) |
• Universal clamp |
(2) |
• Protective glasses |
(1) |
• Condenser |
(1) |
• Bosshead |
(2) |
Chemicals and Other Materials |
|
• Erlenmeyer flask, 100 mL |
(1) |
• Rubber stopper, with one hole |
(1) |
• Sulphuric acid, concentrated |
|
• Separatory funnel |
(1) |
• Rubber stopper, with two holes (1) |
• Sodium Acetate, dry |
|
|
• Graduated cylinder, 10 mL |
(1) |
• Rubber tubing, 50 cm |
(2) |
• Ice |
|
• Thermometer |
(1) |
• Burner |
(1) |
• Table salt |
|
• Crystallising dish |
(1) |
•Tripod |
(1) |
• Pieces of porcelain |
|
• Support base |
(2) |
• Wire gauze |
(1) |
|
|
PRE-LAB DISCUSSION
Acetic acid is the principal ingredient of vinegar which has 4-5% acetic acid and water. The formula of acetic is
Pure acetic acid has a penetrating odour and produces
painful burns. It is an excellent solvent for many organic and some inorganic compounds.
Acetic acid is an important starting substance for making textile fibres, vinyl plastics and other chemicals. It is produced by the oxidation of aceteldehyde, by the fermentation of fruit juices and by the oxidation of alcohol in contact with air in the presence of bacterium acetic.
PROCEDURE
Set-up
—Insert the thermometer and the separatory funnel into the bores of the rubber stopper.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
—Put the distillation flask on the tripod and fix it on the support rod with a universal clamp as in the Figure.
—Weigh 20 g of sodium acetate and dry it by heating in the evaporating dish. Then put it into the distillation flask.
Note: Sodium acetate must be dry.
—Close the mouth of the distillation flask with the stopper connected with thermometer and separatory funnel as in the Figure
—Close the stopcock of the separatory funnel. Measure 10 mL of sulphuric acid with graduated cylinder then put it into the separatory funnel.
Caution: Sulphuric acid is very corrosive. Do not swallow or let it to contact your skin.
—Connect the tube of the distillation flask with the condenser with the help of rubber stopper as seen in the Figure.
—Fix the condenser on the second support rod so that it is held at slight angle.
—Fill the two third of the crystallising dish with crushed ice and add 10 spatulas of table salt.
—Attach the right-angled tube to the outlet of the condenser with the help of a rubber tubing and place the other end of the right-angled tube in the erlenmeyer flask which is in the ice bath as seen in the Figure.
—Connect lower side tube of the condenser to the tap and upper one to drain.
—Wear protective glasses.
Experiment – 73 How can acetic acid be obtained?
186

Procedure |
Figure |
1.Ignite the burner and heat the distillation flask.
2.While heating, open the stopcock of the separatory funnel and add the sulphuric acid into the distillation flask drop by drop. Note your observation in “Observations and Data Tables”.
Note: Hold the temperature between 112 - 118 °C while heating.
3.Extinguish the burner when enough distillate is collected. Examine the appearance and smell of the product in the erlenmeyer flask. Note your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations on heating the distillation flask.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on the distillate.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Why did you add sulphuric acid drop by drop in the experiment?.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Is the vinegar strong or weak acid? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the reaction equation for the reactions occurred during the preparation of acetic acid?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Which substances are used in the production of vinegar in industry? Explain the production method of one of them.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 73 How can acetic acid be obtained? |
187 |
|

What happens when carboxcylic acids react with alcohols? (Esterification)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare ethyl acetate from acetic acid and ethyl alcohol.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Beaker, 250 mL |
(1) |
•Tripod |
(1) |
• Sulphuric acid, concentrated |
• Test tube |
(2) |
• Test tube rack |
(1) |
• Acetic acid |
• Graduated cylinder, 10 mL |
(1) |
• Wire gauze |
(1) |
• Ethyl alcohol |
• Burner |
(1) |
• Protective glasses |
(1) |
• Distilled water |
PRE-LAB DISCUSSION
The general formula of esters is |
. They have |
pleasant odour. They are responsible for the flavour and fra-
grance of many fruits and hovers such as ethyl formate
(rum), n-pentyl acetate (banana), and octyl acetate (orange).
Carboxylic acids react with alcohols to form esters through a
condensation reaction known as esterification;
Esterification reactions occur in the presence of an acid as a catalyst. This reaction is an equilibrium reaction. The use of an excess amount of one of reactants or removing of water from the reaction medium will increase the produced amount of ester.
PROCEDURE
Set-up |
Figure |
—Measure 2 mL of concentrated acetic acid and 2 mL of ethyl alcohol with the graduated cylinder. Place them into a test tube.
Caution: The ethanol is highly inflammable. Extinguish all open flames.
—Measure 0.5 mL of concentrated sulphuric acid into the tube and shake the mixture.
Caution: Sulphuric acid is highly corrosive. Wash splashes off the skin with copious water.
— Wear protective glasses.
Procedure
1.Half fill the beaker with water and place it on the tripod. Ignite the burner and heat the water. When the water starts boiling extinguish the burner flame.
2.Place the test tube into the hot water for about 5 minutes. Note your observations in “Observations and Data Tables”.
Experiment – 74 What happens when carboxcylic acids react with alcohols? (Esterification)
188

Note: If the water in the beaker is cold, re-heat it carefully. The ethanol is highly inflammable
3.Take the test tube out and place into test tube rack. Allow it to cool.
4.Half fill another test tube with distilled water and pour the cooled mixture into it. Test the smell of the resultant solution and record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations on heating the mixture.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Carefully test the smell of the product which have been formed.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the equation for the reaction.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How can you determine that the product is an ester? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Complete the following reactions and write the names of the products.
a.
....................................................................................................................
b.
........................................................................................................
4.What is the role of sulphuric acid in the experiment? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 74 What happens when carboxcylic acids react with alcohols? (Esterification) |
189 |
|

Date : ...............................................................
Partners : ...............................................................
...............................................................
Can aspirin be produced in laboratory? Grade : ...............................................................
PURPOSE : To synthesise aspirin from salicylic acid and acetic acid.
EQUIPMENT and MATERIALS:
Equipment |
|
• Stirring rod |
(1) |
• Protective glasses |
(1) |
• Beaker, 250 mL |
(2) |
• Burner |
(1) |
Chemicals and Other Materials |
|
• Beaker, 100 mL |
(1) |
•Tripod |
(1) |
• Sulphuric acid, concentrated |
|
• Erlenmeyer flask, 100 mL |
(1) |
• Wire gauze |
(1) |
• Salicylic acid |
|
• Filtering flask |
(1) |
• Support base |
(1) |
• Acetic acid |
|
• Buchner funnel |
(1) |
• Support rod |
(1) |
• Distilled water |
|
• Graduated cylinder, 10 mL |
(1) |
• Bosshead |
(1) |
• Filter paper |
|
• Dropper |
(1) |
• Universal clamp |
(1) |
• Ice |
|
PRE-LAB DISCUSSION
Methyl benzoate and methyl salicylate are two of the aromatic esters used in the manufacturing of perfumes and flavouring agents. Methyl salicylate smells and tastes like wintergreen. In general, salicylates are pyretics and analgesic which relieve certain rheumatism. Aspirin is the most common salicylate used in medicine today.
PROCEDURE
Set-up
—Fill the two third of the 250 mL beaker with tap water. Place the beaker on the wire gauze as in the Figure.
—Assemble the buchner funnel with rubber stopper, then close the mouth of the filtering flask with the rubber stopper.
Note: Wet the glassware with glycerol before rubber connections.
—Connect the side tube of the filtering flask to the vacuum filter pump with the rubber tubing.
—Prepare ice bath in 250 mL beaker and ice water in a 100 mL beaker.
—Wear protective glasses.
Procedure
1.Weigh 3 g of salicylic acid with balance, then put it into the 100 mL erlenmeyer flask.
2.Measure 5 mL of acetic acid with 10 mL graduated cylinder, then add it into erlenmeyer flask carefully.
3.While stirring regularly, add 8 drops of sulphuric acid into erlenmeyer flask with a dropper gradually.
Caution: Sulphuric acid is highly corrosive. Wash splashes off the skin with copious water
4.Swirl the mixture in the erlenmeyer flask for about 5 minutes.
5.Place the erlenmeyer flask into the beaker and clamp it on the support rod.
Note: Be sure that the bottom of the erlenmeyer flask does not touch the bottom of the beaker.
6.Ignite the burner and heat the contents of the erlenmeyer flask by boiling the water in the beaker for 15 minutes as in the Figure-1.
7.Extinguish the burner, then take the erlenmeyer out from the water bath.
Experiment – 75 Can aspirin be produced in laboratory?
190

8.While the contents of the erlenmeyer is still hot, carefully add 5 mL of ice water into the erlenmeyer flask at once with graduated cylinder. Record your observations in “Observations and Data Tables”.
9.When the crystallisation slow down, add 40 mL of water into the erlenmeyer flask
and place it into the ice-bath in the beaker for 2 minutes.
Note: If lumps of solid is formed in the erlenmeyer, break them with stirring rod.
10.Take the erlenmeyer out from the ice-bath. Filter the mixture through the buchner funnel by vacuum filtration as in the Figure-2.
11.Rinse the product in the buchner funnel with 15 mL of ice water.
12.Transfer the product in the buchner funnel on a sheet of dry filter paper. Allow it to dry completely. Record your observations on the product in “Observations and Data Tables”.
Caution: Do not attempt to swallow your product, It is not as safe as the commercial aspirin to use.
13.Take a few crystals of your aspirin on a piece of wet blue litmus paper. Record your observations in “Observations and Data Tables”.
Figure-2
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations on the litmus paper test.
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.What kind of impurities may be present in your aspirin?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.What is the function of the sulphuric acid in the formation of aspirin?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Aspirin passes through stomach without changing, but it is absorbed in the intestines? Research the reason.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 75 Can aspirin be produced in laboratory? |
191 |
|
How can soap be produced?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare soap from fat.
EQUIPMENT and MATERIALS:
Equipment |
|
• Stirring rod |
(1) |
Chemicals and Other Materials |
|
• Beaker, 250 mL |
(2) |
• Spatula |
(1) |
• Fat, (or oil) |
|
• Beaker, 100 mL |
(1) |
• Rubber stopper, without hole |
(1) |
• Ethyl alcohol |
|
• Graduated cylinder, 10 mL |
(1) |
• Balance |
(1) |
• Sodium hydroxide, 25% |
|
• Erlenmeyer flask, 200 mL |
(1) |
• Burner |
(1) |
• Table salt solution, concentrated |
|
• Funnel |
(1) |
• Tripod |
(1) |
• Distilled water |
|
• Test Tube |
(1) |
• Wire gauze |
(1) |
• Filter paper |
|
• Dropper |
(1) |
• Protective glasses |
(1) |
||
|
PRE-LAB DISCUSSION
Soaps are sodium and potassium salts of fatty acids, particularly stearic, palmitic and oleic acids. Animal and vegetable oils and fats, from which soaps are prepared, consist essentially of the glyceryl esters of these acids. In soap manufacture the oil or fat is heated with dilute sodium hydroxide solution in large vast. When hydrolysis is complete the soap is precipitated from solution by addition of sodium chloride
salt. It is then treated, as required, with perfumes, etc. and made into tablets.
Soaps have a nonpolar chain and an ionic carboxylate group. The hydrocarbon end of a soap molecule is attracted by dirt, oil or grease particles, and the ionic end is attracted by water. Then the dirt particles are washed away in water.
PROCEDURE
Set-up
—Put 3 spoonfuls of fat in the 250 mL beaker, then stand the beaker on the wire gauze as in the Figure.
—Prepare 25% sodium hydroxide solution in 100 mL beaker.
Note: To prepare 25% sodium hydroxide solution, dissolve 34 g of sodium hydroxide in 100 mL distilled water.
—Prepare concentrated table salt solution in 250 mL beaker.
Note: To prepare concentrated table salt solution, dissolve 15 g of table salt into 100 mL distilled water.
— Wear protective glasses.
Procedure
1.Ignite the burner and heat the beaker carefully with small flame so that the fat just melts.
Caution: Do not heat fat so much. At high temperatures, sodium hydroxide tends to spit strongly.
2.Add 10 drops of ethyl alcohol and 5 mL of distilled water into the beaker.
Caution: When handling alcohols, extinguish all open flames. Alcohols are highly inflammable.
3. Add, little by little, 10 mL of 25% sodium hydroxide so-
lution and heat for a further 10 minutes by stirring with stirring rod. Record your observations in “Observations and Data Tables”.
Caution: Sodium hydroxide is highly corrosive. Wear protective gloves.
Note: During heating, replace evaporated water by the careful addition of distilled water.
4.Extinguish the burner flame and pour the mixture quickly into the table salt solution. Stir the mixture for 2-3 minutes with stirring rod.
5.Allow the mixture to cool and filter it. Wash the solid residue in the funnel with a little distilled water and allow the solid to dry.
6.Pick up a little solid with spatula and put it into a test tube.
7.Add distilled water into the test tube. So that water level is over solid. Stopper the test tube and shake it vigorously. Record your observations in “Observations and Data Tables”.
Note: Different fat and oil can be used and different procedure can be applied in different group. Perfumes and dye also can be added into your soap.
Experiment – 76 How can soap be produced?
192
