
experment book
.pdf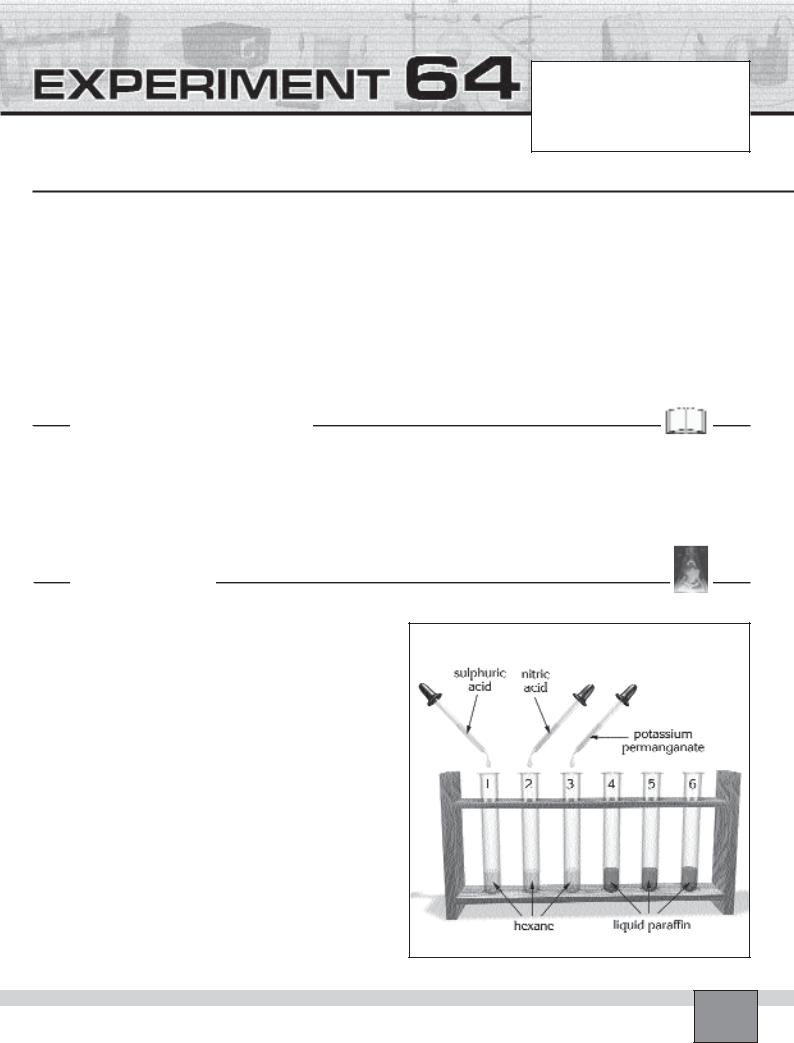
Do alkanes react with concentrated acids and permanganate solutions?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To test the reactivity of alkanes toward acids and oxidising agents.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
|
• Test tubes |
(6) |
• Hexane |
• Potassium permanganate, 5% |
• Test tube rack |
(1) |
• Liquid paraffin |
• Sodium carbonate, 10% |
• Dropper |
(3) |
• Sulphuric acid, concentrated |
• Litmus paper |
• Protective glasses |
(1) |
• Nitric acid, concentrated |
|
PRE-LAB DISCUSSION
Alkanes are very unreactive. They do not react with strong acids such as sulphuric acid and nitric acid. They do not react even with the strong oxidising agent, such as potassium permanganate. This is clearly depicted in the designation “saturated” hydrocarbons. They do not accept any further
elements. Alkanes are formed of C-C and C-H single bonds, which are very difficult to attack. These bonds are very stable and non-polar so that other particles are not attracted and find no target.
PROCEDURE
Set up |
Figure |
—Put six test tubes in the test tube rack and number them with marker pan.
—Fill the test tubes from 1 to 3 with hexane up to a height of about 1 cm.
Caution: Extinguish all flames before pouring hexane. Because it is highly inflammable. After using hexane, close its bottle and put it far away from working area.
—Fill the test tubes from 4 to 6 with liquid paraffin up to a height of about 1 cm.
—Wear protective glass.
Procedure
1.Add a drop of concentrated sulphuric acid into the test tube 1 and record your observations in the Table in “Observations and Data Tables”.
Caution: Sulphuric acid and nitric acid are highly corrosive. Immediately wash any splashes on your skin with water.
2.Add a drop of concentrated nitric acid into the test tube 2 and record your observations in the Table in “Observations and Data Tables”.
Experiment – 64 Do alkanes react with concentrated acids and permanganate solutions? |
163 |
|

3.Add a few drops of fresh alkaline potassium permanganate solution into the test tube 3 and record your observations in the Table in “Observations and Data Tables”.
Note: Use different dropper for each solution added .
Note: To prepare alkaline potassium permanganate solution add 5% potassium permanganate solution to 10% sodium carbonate solution to get a light violet colour.
4.Repeat the steps from 1 to 3 for the liquid paraffin in the test tubes from 4 to 6. Record your observations in the Table in “Observations and Data Tables”.
5.Test the acidity of the all solutions with litmus paper, then Record the result in the Table in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Record your test results in the Table below.
Test tubes |
|
Reaction / No reaction |
|
Acidic / Basic |
|
|
|
|
|
Hexane + sulphuric acid |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
|
|
|
|
|
Hexane + nitric acid |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
|
|
|
|
|
Hexane + permanganate solution |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
|
|
|
|
|
Paraffin + sulphuric acid |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
|
|
|
|
|
Paraffin + nitric acid |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
|
|
|
|
|
Paraffin + permanganate solution |
|
|
|
......................................................... |
|
|
......................................................... |
|
|
EVALUATIONS AND CONCLUSIONS 


1.Draw a conclusion from the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Why the reactivity of alkanes is low? Give reasons.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What are the characteristic properties of alkanes? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 64 Do alkanes react with concentrated acids and permanganate solutions?
164
How is ethylene prepared? and what are the properties of ethylene?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare ethylene and examine some of its properties.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Test tube |
(3) |
Universal clamp |
(3) |
• Ethyl alcohol |
• Crystallising dish |
(1) |
• Bosshead |
(3) |
• Aluminium oxide, dried |
• Glass tube, right-angled |
(1) |
• Rubber stopper, with one hole |
(1) |
• Sand, dry and fine |
• Delivery tube, with tip |
(1) |
• Rubber stopper, without hole |
(2) |
• Potassium permanganate, 0,01M |
• Test tube rack |
(1) |
• Rubber tubing, 20 cm |
(1) |
|
• Support base |
(2) |
• Burner |
(1) |
|
• Support rod |
(2) |
• Protective glasses |
(1) |
|
PRE-LAB DISCUSSION
Hydrocarbon molecules that contain a double bond are called as alkenes. The two carbon atoms linked by a double bond, one (sigma) bond and one bond.
Ethene (Ethylene), C2H4, is the simplest alkene. Each carbon atom in ethylene has a trigonal planer structure.
The bond angles at each carbon are approximately 120°. Each carbon atom is attached to only three atoms by sigma bonds. The unhybridised 2p orbitals of carbon atoms may also overlap to form a second bond (a pi( ) bond)
PROCEDURE
Set up
—Put the crystallising dish on the bench and fill the two third of it with tap water.
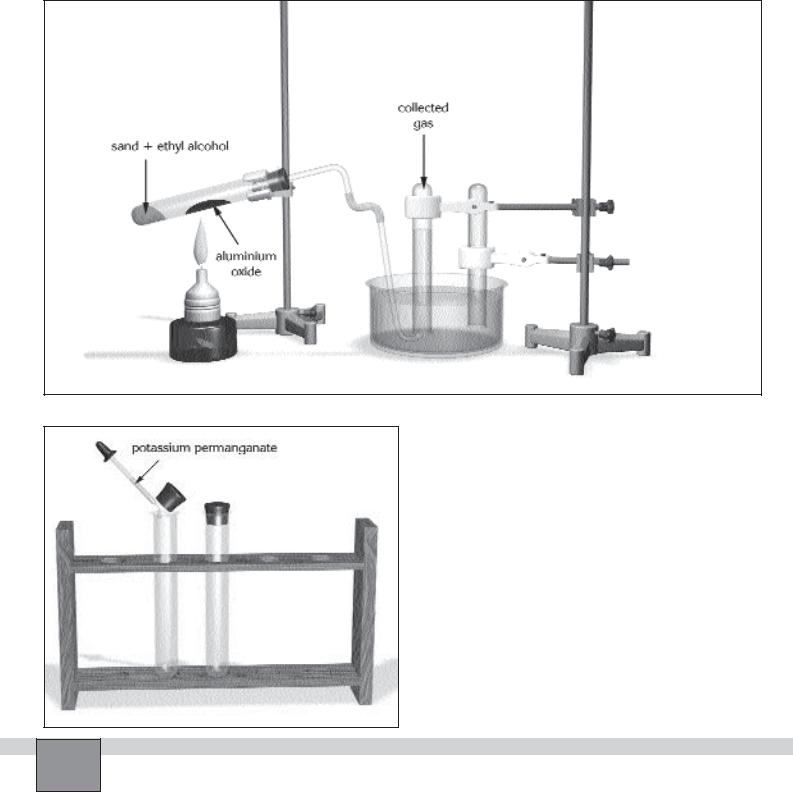
—Fill a test tube with tap water. Close the mouth of the tube with your thumb (or a piece of paper), then sink into the water in the crystallising dish. Open the mouth of the tube. Proceed the same process for another test tube. Fix the tubes on the support rod as in the Figure.
—Connect the short arm of the right-angled glass tube to the delivery tube with tip by a piece of rubber tubing and connect the long arm of the right-angled glass tube to the rubber stopper.
Note: Lubricate glass-rubber connections with glycerol before connection.
—Take a test tube and fill it with standard sand up to about 3 cm height.
—Pour ethanol on the sand so that it is completely wet in ethanol.
Note: Make sure that none of the sand overlies.
—Fix the test tube on the stand at a very slight angle as seen in the Figure.
—Place 3 spatulas of aluminium oxide in the middle of the test tube.
Note: Before the experiment aluminium oxide must be brought to glowing or to be dried overnight at 200°C in an owen.
—Assemble the apparatus as in the Figure
—Wear protective glasses.
Experiment – 65 How is ethylene prepared? and what are the properties of ethylene? |
165 |
|
Procedure
1.Ignite the burner and heat the front of the test tube then heat the aluminium oxide to a red-glow. Record your observations in “Observations and Data Tables”.
Note: In general, heating aluminium oxide is sufficient to cause the ethanol to evaporate. If necessary, heat the ethanol carefully for a short time so that it is briefly warmed.
Caution: Do not heat the tube too much since it contains explosive gases.
Figure-1
2.After about 30 second slip the tip of the delivery tube to the mouth of the inverted test tube in the water in the crystallising dish.
3.When the inverted test tube is full of evolved gas. Slip
the tip of the delivery tube to the mouth of the second inverted test tube.
4.Close the test tubes with rubber stopper under water, then place them in the test tube rack.
Caution: Place the test tube rack as far away as possible from the burner.
Figure-2
5. Remove the tip of the delivery tube from the water and extinguish the burner.
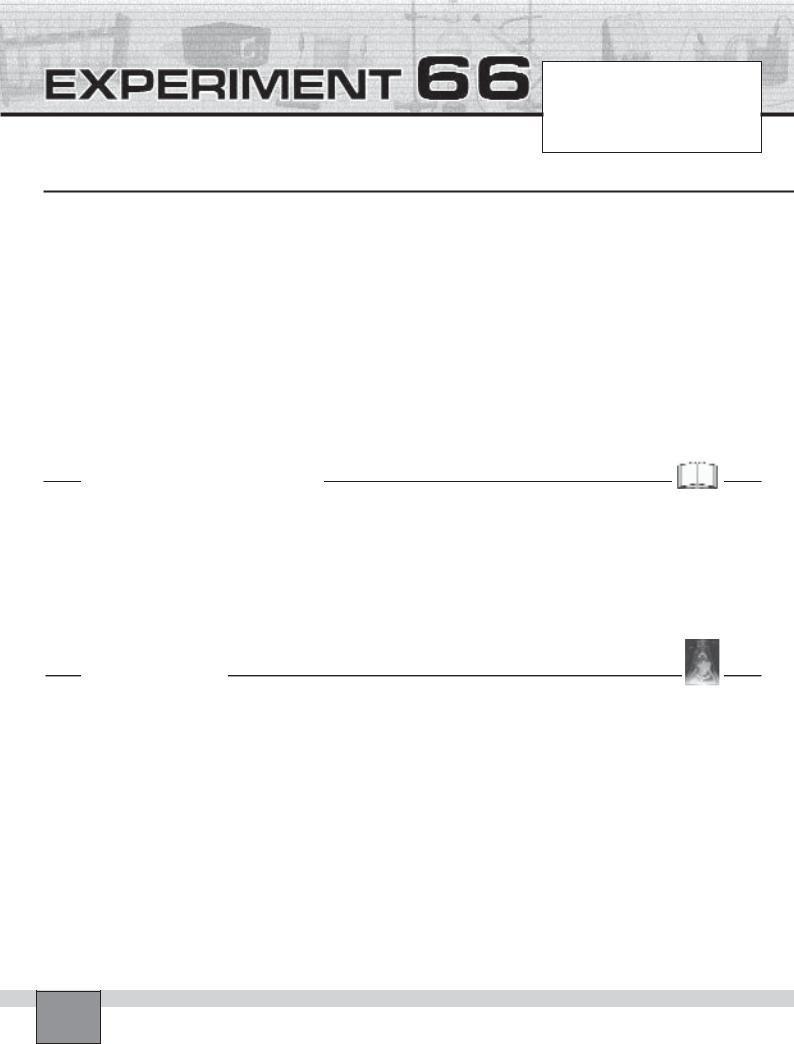
6. Take one of the test tubes containing collected gas and put a few drops of potassium permanganate solution in the test tube.
Note: Lift the stopper of the test tube slightly and close it again immediately.
7. Shake the test tube vigorously and record your observations in “Observations and Data Tables”.
8. Ignite the burner. Hold the second test tube containing collected gas upside down so that the mouth of the test tube at the flame of the burner, then remove the stopper. Record your observations in “Observations and Data Tables”.
Caution: Air the room well after the experiment..
Experiment – 65 How is ethylene prepared? and what are the properties of ethylene?
166

OBSERVATIONS AND DATA TABLES 


1.Note your observations on the heating of the aluminium oxide.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations you made;
on the addition of Permanganate : ....................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on the burning of the collected gas : .................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the reaction equation for the preparation reaction of ethylene.
...........................................................................................................................................................................................
2.Write the reaction equation occurred in the addition of permanganate solution.
...........................................................................................................................................................................................
3.Give reasons for the reactivity behaviour of the alkenes which you have determined.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Write the observed properties of the ethylene and look up the missing properties in your text book.
Chemical symbol: ...................... Colour : ...................... Melting point : ...................... Boiling point : ......................
Other properties :
...........................................................................................................................................................................................
Usage : ..............................................................................................................................................................................
Experiment – 65 How is ethylene prepared? and what are the properties of ethylene? |
167 |
|
How is acetylene prepared? and what are the properties of acetylene?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare acetylene and examine some of its properties.
EQUIPMENT and MATERIALS:
Equipment |
|
• Universal clamp |
(3) |
Chemicals and Other Materials |
• Test tube |
(3) |
• Bosshead |
(3) |
• Calcium carbide |
• Crystallising dish |
(1) |
• Rubber stopper, with one hole |
(1) |
• Potassium permanganate, 0,01M |
• Glass tube, right-angled |
(1) |
• Rubber stopper, without hole |
(2) |
• Copper(I) chloride, |
• Delivery tube, with tip |
(1) |
• Rubber tubing, 20 cm |
(1) |
• Ammonia solution, concentrated |
• Test tube rack |
(1) |
• Burner |
(1) |
• Distilled water |
• Support base |
(2) |
• Protective glasses |
(1) |
|
• Support rod |
(2) |
|
|
|
PRE-LAB DISCUSSION
Alkynes have triple bond in their molecule, they have the general formula CnH2n-2. It has linear geometric shape. Be-
cause of this linear geometric shape, it has no cis-trans isomers. Combustion of alkynes gives more energy than the others because of high percentage of carbon in structure. Mostly known alkyne which is acetylene, is produced industrly from limestone and coke.
Many chemical reactions of alkynes show similar characteristic properties as alkanes. (Addition reaction hydrogenation, halogenation, and etc.) The identification method of alkynes is the reaction of alkynes with Tollen’s reagent. This reaction gives white precipitation. Reaction of alkynes with fehling’s reagent gives red precipitation.
PROCEDURE
Set up
—Put the crystallising dish on the bench and fill the two third of it with tap water.
—Fill a test tube with tap water. Close the mouth of the tube with your thumb (or a piece of paper), then sink into the water into the crystallising dish. Open the mouth of the tube. Proceed the same process for another test tube. Fix them on the stand rod as in the Figure-1.
—Connect the short arm of the right-angled glass tube to the delivery tube with tip by a piece of rubber tubing and connect the long arm of the right-angled glass tube to the rubber stopper.
Note: Lubricate glass-rubber connections with glycerol before connection.
—Fix another test tube on the stand as seen in the Figure 1.
—Put a spatula of calcium carbide in the test tube.
—Assemble the apparatus as in the Figure-1.
—Wear protective glasses.
Procedure
1.Pour distilled water onto the calcium carbide in the test tube to the height of about 2 cm. Close the tube with its stopper.
Caution: Do not heat the tube since it contains explosive gas.
2.After about 15 seconds slip the tip of the delivery tube to the mouth of the inverted test tube in the water.
3.When the inverted test tube is full of evolved gas. Slip the tip of the delivery tube to the mouth of the second inverted test tube.
Experiment – 66 How is acetylene prepared? and what are the properties of acetylene?
168
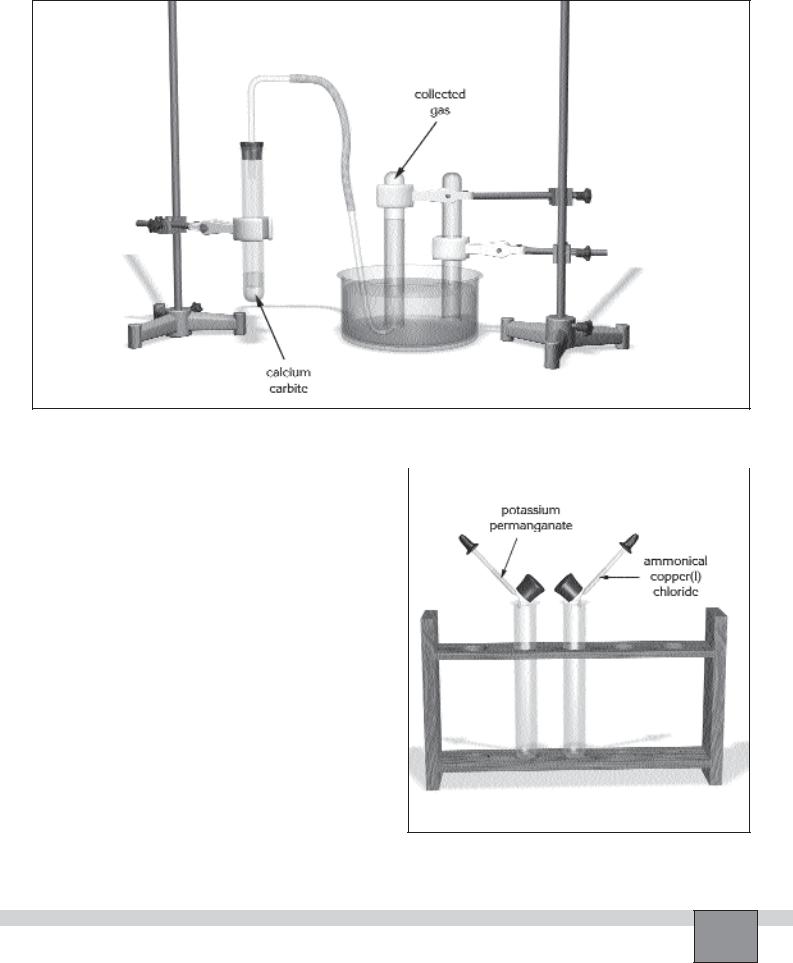
Figure-1
4. Close the test tubes with rubber stopper under water, |
Figure-2 |
then place them in the test tube rack. |
|
|
Caution: Place the test tube rack as far away as possible from the burner and working area
5.Take one of the test tube containing collected gas and put a few drops of potassium permanganate solution in the test tube as in the Figure-2.
Note: Lift the stopper of the test tube slightly and close it again immediately.
6.Shake the test tube vigorously and record your observations in “Observations and Data Tables”.
7.Take the second test tube containing collected gas and put a few drops of ammoniacal copper(I) chloride solution (Fehling’s reagent) in the test tube.
Preparation ammoniacal copper(I) chloride solution: Mix 25 mL of copper(I) chloride and 5 mL concentrated ammonia solution.
Note: Lift the stopper of the test tube slightly and close it again immediately.
8.Shake the test tube vigorously and record your observations in “Observations and Data Tables”.
Caution: Air the room well after the experiment.
Experiment – 66 How is acetylene prepared? and what are the properties of acetylene? |
169 |
|

OBSERVATIONS AND DATA TABLES 


1.Note your observations on addition of water.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Note your observations you made;
on the addition of permanganate : ....................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on the addition of Fehling’s solution : ...............................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Write the reaction equation;
For the production of acetylene : .........................................................................................................................................
For the acetylene-permanganate reaction : ........................................................................................................................
For the acetylene-Fehling’s reagent reaction : ..................................................................................................................
2.What are the common properties of acetylene and the other alkynes?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the observed properties of the acetylene and look up the missing properties in your text book.
Chemical symbol: ...................... |
Colour : ...................... |
Melting point : ...................... |
Boiling point : ...................... |
Other properties : ................................................................................................................................................................ |
|
|
|
Usage : ............................................................................................................................................................................... |
|
|
|
Experiment – 66 How is acetylene prepared? and what are the properties of acetylene?
170

How does the structure of alcohols affect their properties and reactions?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To examine the properties of some monohydric and polyhydric alcohols. To examine the reactions of primary, secondary and tertiary alcohols.
EQUIPMENT and MATERIALS:
Part-A |
|
|
Part-B |
|
Equipment |
|
Equipment |
|
|
• Test tube |
(7) |
• Test tube, small |
(7) |
|
• Test tube rack |
(1) |
• Test tube rack |
(1) |
|
• Dropper |
(1) |
• Dropper |
(1) |
|
• Evaporating dish |
(1) |
• Rubber stopper, without hole |
(3) |
|
• Protective glasses |
(1) |
• Protective glasses |
(1) |
|
Chemicals and Other Materials |
Chemicals and Other Materials |
• Ethanol |
• Primary butanol |
• Glycerol |
• Secondary butanol |
• Primary butanol |
• Tertiary butanol |
• Sodium hydroxide, 2M |
• Potassium permanganate, 0.01 M |
• Copper(II) sulphate, 0.02M |
• Hydrochloric acid, 12 M |
•Potassium permanganate, 0.01M
•Wood splint
PRE-LAB DISCUSSION
Organic compounds which contain hydroxyl groups are called alcohols. Their general formula is CnH2n+1OH or
CnH2n+2O. Mostly used alcohols, in daily life are ethyl alcohol, glycol, and glycerol.
Ethyl alcohol can be produced by the fermentation of aqueous solutions of simple carbohydrates.
yeast
C12H22O11 + H2O 4C2H5OH + 4CO2
(sugar)
Ethanol is used in industry as a solvent, it is also used as ingredient of fermented alcoholic beverages and a tropical antiseptic.
Alcohols which have 1-11 carbons are colourless. Solubility of alcohols in water decreases when the carbon chain in alcohol increases.
Oxidation of primary alcohols forms aldehyde.
Oxidation of secondary alcohols forms ketone.
Tertiary alcohols are not oxidised.
For separation of alcohol from the other organic compounds, reaction of it with active metals is used.
Experiment – 67 How does the structure of alcohols affect their properties and reactions? |
171 |
|
PROCEDURE
Part-A
Set up
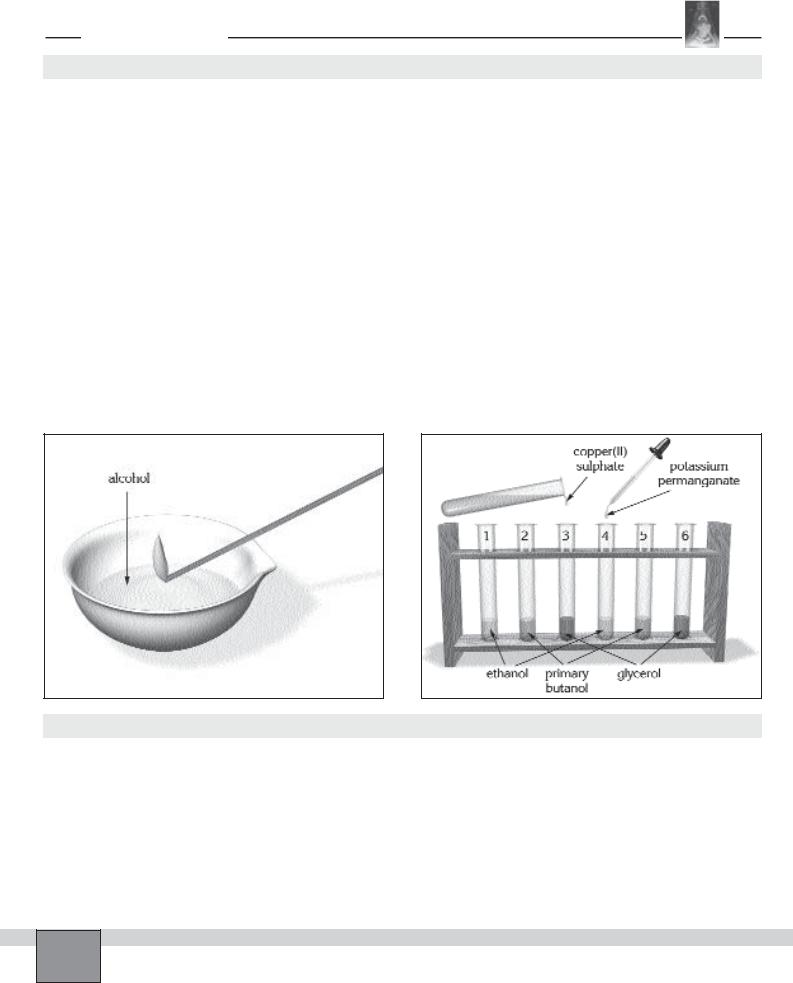
—Place 6 test tubes in the test tube rack and number them from 1 to 6 with marker pen.
—To height of about 1 cm;
Fill the test tube 1 and 4 with ethanol
Fill the test tube 2 and 5 with primary butanol
Fill the test tube 3 and 6 with glycerol
Caution: Butanol is harmful to health. Do not inhale or swallow it.
Caution: Some of the substances are highly inflammable. Extinguish all open flames while handling them.
— Wear protective glasses.
Procedure
1.Examine the appearance of the alcohols, then note your observations in “Observations and Data Tables”.
2.Take a little ethanol with a dropper into an evaporating dish and try to ignite it with a burning wood splint. Record your observations in “Observations and Data
Figure-1
Part-B
Set up
—Take 6 clean small test tubes and place them in the test tube rack then number them from 1 to 6.
—To height of about 1 cm
Fill the test tube 1 and 4 with primary butanol
Fill the test tube 2 and 5 with secondary butanol
Fill the test tube 3 and 6 with tertiary butanol
Caution : Butanol is harmful to health. Do not inhale or swallow it.
Tables”.
3.Repeat step 2 for primary butanol and glycerol.
4.Take a little ethanol and try to dissolve in distilled water. Record your observations in “Observations and Data Tables”.
5.Repeat step 4 for primary butanol and glycerol.
6.Add triple amount of copper(II) sulphate solution into test tubes 1, 2 and 3 then add 2 mL sodium hydroxide solution into them.
Note: The addition of sodium hydroxide must be stopped as soon as blue colour forms.
7.Mix the solutions by shaking carefully and record any colour change in the colour of the mixtures in “Observations and Data Tables”.
8.Add several drops of potassium permanganate solution to the test tubes 4, 5 and 6. Record any colour changes in the colour of the permanganate solutions in about five minutes in “Observations and Data Tables”.
Figure-2
— Wear protective glasses.
Procedure
1.Add 5 mL 12 M hydrochloric acid solution into test tube 1 and close the mouth of it then mix the liquids by shaking the test tube for a minute. Record your observations in “Observations and Data Tables”.
Caution : Handle acids and bases always with great care. Do not let them split on your skin. If that happens, wash immediately with water.
Experiment – 67 How does the structure of alcohols affect their properties and reactions?
172
