
experment book
.pdf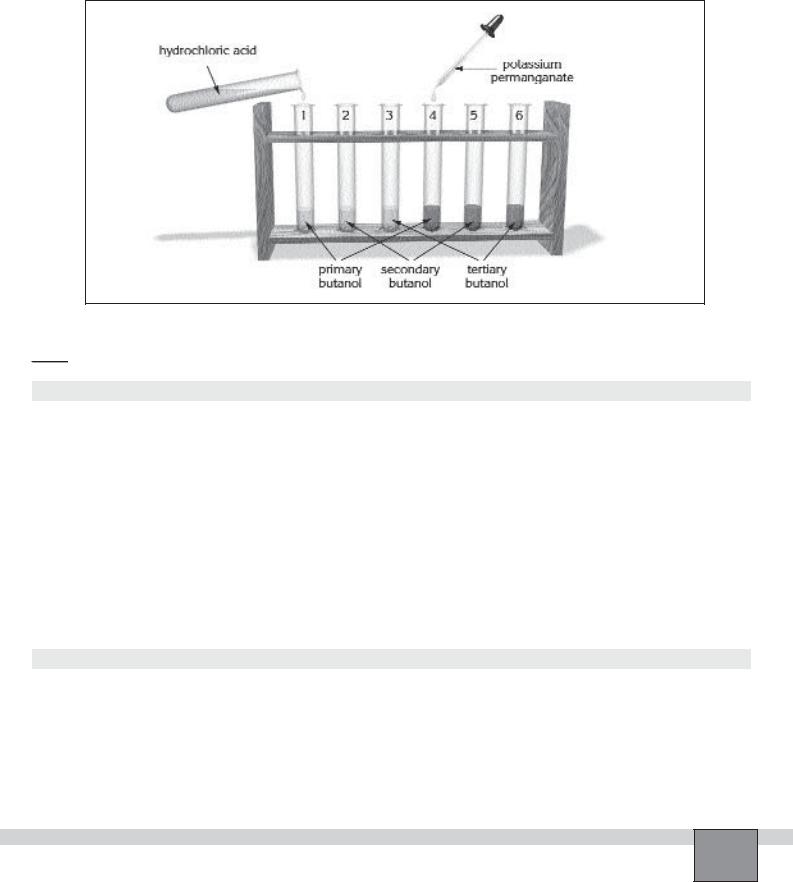
2. |
Repeat step 1 for test tube 2 and 3. |
Wait for about five minutes then record your observa- |
3. |
Add several drops of potassium permanganate solution |
tions on the colour of the permanganate solution in |
|
into test tube 4 and close the mouth of it with rubber |
“Observations and Data Tables”. |
|
stopper then mix the liquids by shaking the test tube. |
4. Repeat step 3 for test tube 5 and 6. |
Figure
OBSERVATIONS AND DATA TABLES 


Part-A
1.Note your observations in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Enter your observations in the Table below.
|
|
|
|
|
|
Solubility in |
|
Addition of cop- |
|
Colour of per- |
Alcohol |
|
Colour |
|
Combustibility |
|
water |
|
per(II) sulphate |
|
manganate |
Ethanol |
|
|
...................... |
...................... |
...................... |
...................... |
||||
|
|
...................... |
||||||||
Primary butanol |
|
|
...................... |
...................... |
...................... |
...................... |
||||
|
|
...................... |
||||||||
Glycerol |
|
|
...................... |
...................... |
...................... |
...................... |
||||
|
|
...................... |
||||||||
Part-B
1.Note your observations below.
Butanols |
|
Colour |
|
Addition of hydrochloric acid |
|
Colour of permanganate |
|
|
|
|
|
|
|
Primary butanol |
|
...................................... |
........................................... |
........................................... |
||
Secondary butanol |
|
...................................... |
........................................... |
........................................... |
||
Tertiary butanol |
|
...................................... |
........................................... |
........................................... |
||
Experiment – 67 How does the structure of alcohols affect their properties and reactions? |
173 |
|

EVALUATIONS AND CONCLUSIONS 


Part-A
1.Draw a conclusion for the part-A of the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How does the number of carbon affect the properties of a primary alcohol?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.How do polyhydric alcohols differ from monohydric alcohols experimentally? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.How can polyhydric and monohydric alcohols be differentiated experimentally?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Part-B
1.Draw a conclusion for the part-B of the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.How can a tertiary alcohol be distinguished experimentally?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.What are the common properties of the isomers of butanol?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 67 How does the structure of alcohols affect their properties and reactions?
174
How is diethyl ether produced in laboratory?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare diethyl ether from ethanol.
EQUIPMENT and MATERIALS:
Equipment |
|
• Bosshead |
(2) |
Chemicals and Other Materials |
• Distillation flask, 500 mL |
(1) |
• Rubber stopper, with one hole |
(3) |
• Ethanol |
• Condenser |
(1) |
• Rubber tubing, 50 cm |
(2) |
• Sulphuric acid, concentrated |
• Erlenmeyer flask, 100 mL |
(1) |
• Burner |
(1) |
• Sodium chloride |
• Separatory funnel |
(1) |
• Tripod |
(1) |
• Ice |
• Crystallising dish |
(1) |
• Wire gauze |
(1) |
|
• Support base |
(2) |
• Spatula |
(1) |
|
• Support rod |
(2) |
• Protective glasses |
(1) |
|
• Universal clamp |
(2) |
|
|
|
PRE-LAB DISCUSSION
The structure of ether is closely related to the alcohols
Replacement of both hydrogen atoms with alkyl group de-
rives ether structure in water molecule. Commonly, production method of ether is dehydration of alcohols.
Ethers are slightly soluble in water, but it is an unusually good solvent for organic compounds. They are colourless, but have characteristic pleasant odours. Their boiling points are lower than alcohol because of absence of hydrogen bond.
PROCEDURE
Set-up
—Insert the separatory funnel into bore of the rubber stopper.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
—Fix the distillation flask on the support rod with a universal clamp as in the Figure.
—Put 50 mL ethanol in the distillation flask and add 50 mL sulphuric acid onto ethanol.
Caution: Handle acids and bases always with great care.
—Assemble the separatory funnel with distillation flask as in the Figure. Close the stopcock of the separatory funnel.
—Fix the condenser on the second support rod so that it
is held at slight angle.
—Fill the two third of the crystallising dish with crushed ice and add 10 spatulas of sodium chloride salt.
—Attach the erlenmeyer flask to the outlet of the condenser with the help of a rubber stopper as in the Figure, then place the erlenmeyer flask in the ice bath.
Note: Boiling point of diethyl ether is quite low. Therefore you must close the mouth of the collecting erlenmeyer with a stopper.
—Connect lower side tube of the condenser to the tap and upper one to drain.
—Place 50 mL ethanol into the separatory funnel.
—Wear protective glasses.
Experiment – 68 How is diethyl ether produced in laboratory? |
175 |
|

Procedure: Figure
1.Ignite the burner and heat the mixture in the distillation flask to boil.
Caution: Since ethanol is highly inflammable, heat the distillation flask carefully.
2.While heating distillation flask on wire gauze add ethanol from separatory funnel slowly.
Note: Pay attention to make the rate of addition of alcohol equal to the rate of formation of ether in the erlenmeyer flask.
3.Continue boiling for 5 more minutes, after addition of all alcohol into distillation flask.
4.Examine the collected diethyl ether and record your observations in “Observations and Data Tables”.
Caution: Do not smell, inhale or swallow obtained diethyl ether. It affects nerve system.
Note: Air the room after experiment
OBSERVATIONS AND DATA TABLES 


1.Note your observations in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.What is the role of sulphuric acid in the experiment?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the reaction equation for the formation of diethly ether.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Summarise the properties of the diethyl ether
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.List several usages of the ethers in different areas.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 68 How is diethyl ether produced in laboratory?
176
How can acetaldehyde be prepared? and what are the properties of aldehydes?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare acetaldehyde by the oxidation of primary alcohol.
To examine its several properties.
EQUIPMENT and MATERIALS:
Equipment |
|
|
|
Chemicals and Other Materials |
• Distillation flask, 500 mL |
(1) |
• Universal clamp |
(2) |
• Sulphuric acid, concentrated |
• Condenser |
(1) |
• Bosshead |
(2) |
• Sodium hydroxide, 2M |
• Erlenmeyer flask, 100 mL |
(1) |
• Rubber stopper, with one hole |
(3) |
• Potassium permanganate, 0.01M |
• Separatory funnel |
(1) |
• Rubber tubing, 50 cm |
(2) |
• Ethanol |
• Beaker, 100 mL |
(2) |
• Burner |
(1) |
• Fehling’s-I and Fehling’s-II |
• Crystallising dish |
(1) |
• Tripod |
(1) |
• Ammoniacal silver nitrate solution |
• Test tubes, small |
(3) |
• Wire gauze |
(1) |
• Potassium dichromate |
• Dropper |
(1) |
• Spatula |
(1) |
• Sodium chloride |
• Support base |
(2) |
• Protective glasses |
(1) |
• Ice |
• Support rod |
(2) |
|
|
• Distilled water |
PRE-LAB DISCUSSION
Compounds containing the carbonyl group are called aldehyde. General formula is  except formaldehyde, which is agas. The simpler aldehyde are colourless liquids. Aldehydes with low molecular weight have sharp odour, but the smell becomes more fragrant as the molecular weight increases. Thus, many of aldehydes are used for their
except formaldehyde, which is agas. The simpler aldehyde are colourless liquids. Aldehydes with low molecular weight have sharp odour, but the smell becomes more fragrant as the molecular weight increases. Thus, many of aldehydes are used for their
flavour or perfumes.
Aldehydes with low molecular weight are soluble in water because of polarity of the carbonyl group. The boiling points of aldehydes are higher than ether and hydrocarbons or than ethers of corresponding molecular weight. They do not form hydrogen bonds. Therefore their boiling points are lower than the alcohols.
Oxidation of aldehyde by K2Cr2O7 or KMnO4 forms carboxylic acids.
+
Some times tollen’s reagent (Ag(NH3)2) is used to detect the presence of an aldehyde or to distinguish between aldehyde and ketone.
Experiment – 69 How can acetaldehyde be prepared? and what are the properties of aldehydes? |
177 |
|

PROCEDURE
Set-up
—Insert the separatory funnel into bore of the rubber stopper.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
—Fix the distillation flask on the support rod with a universal clamp as in the Figure.
—Prepare a solution with 10 mL concentrated sulphuric acid and 25 mL distilled water in a beaker, then pour it into the distillation flask.
Caution: Handle acids and bases always with great care.
—Assemble the separatory funnel with distillation flask as in the Figure. Close the stopcock of the separatory funnel.
—Mix 20 g of powdered potassium dichromate, 25 mL distilled water and 20 mL ethanol in another beaker. Pour the mixture into the separatory funnel
—Connect the tube of the distillation flask with the condenser with the help of rubber stopper as seen in the Figure.
—Fix the condenser on the second support rod so that it is held at slight angle.
—Fill the two third of the crystallising dish with crushed ice and add 10 spatulas of sodium chloride salt.
—Place the erlenmeyer flask in the ice bath as in the Figure.
—Connect lower side tube of the condenser to the tap and upper one to drain.
—Wear protective glasses.
Procedure:
1.Ignite the burner and heat the solution gently in the distillation flask until it boils.
2.Open the stopcock of the separatory funnel and let the solution falls into the distillation flask dropwise while the solution in the flask boils. Record your observations in “Observations and Data Tables”.
3.After the solution in the separatory funnel is used up, boil the solution for about five minutes.
Note: Close the stopcock of the separatory funnel when the solution is used up.
4.Extinguish the burner and leave the collected acetaldehyde in the ice bath in the erlenmeyer flask.
Caution: Do not inhale or swallow acetaldehyde
5.Place a test tube in the test tube rack and put about several drops of the acetaldeyhde into the test tube.
6.First add 2 drops of concentrated sulphuric acid, then add 2 drops of potassium permanganate solution onto the acetaldehyde. Record your observations on the colour of the permanganate solution in “Observations and Data Tables”.
7.Place another test tube in the test tube rack and put about several drops of the acetaldeyhde into the test tube.
8.First add about 5 mL of ammoniacal silver nitrate solution, then add a little amount of sodium hydroxide solution onto the acetaldehyde. Record your observations in “Observations and Data Tables”.
9.Place the third test tube in the test tube rack and
first put about 3 mL of Fehling’s-I solution, then 3 mL of Fehling’s-II solution. Make the solution basic with sodium hydroxide solution.
Note: Stop the addition of sodium hydroxide solution when the colour of the mixture is dark-blue.
10.Add several drops acetaldehyde onto Fehling’s solutions. Record your observations in “Observations and Data Tables”.
Figure
Experiment – 69 How can acetaldehyde be prepared? and what are the properties of aldehydes?
178
OBSERVATIONS AND DATA TABLES 


1.Note your observations in general.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Enter your observations
on the addition of potassium permanganate solution : .......................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on the addition of ammoniacal silver nitrate solution : ........................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on the addition of Fehling’s solutions : ................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.What is the role of the potassium dichromate in the production of acetaldehyde?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Give the properties of acetaldehyde that you observed in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Give the non-observed properties of aldehydes in the experiment.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.How can alkanals be distinguished from alkanones?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Give two other methods for the production of acetaldehyde.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
6.For what purposes aldehydes are used in industry? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 69 How can acetaldehyde be prepared? and what are the properties of aldehydes? |
179 |
|

|
|
|
|
|
|
Date |
...............................................................: |
|
|
|
|
|
|
|
Partners : ............................................................... |
|
|
|
|
|
|
|
|
|
............................................................... |
|
|
|
|
|
|
|
|
|
|
|
How is acetone produced technically? |
|
Grade |
: ............................................................... |
|
|||
|
|
|
|
|||||
|
|
|
|
|||||
|
PURPOSE : To produce acetone from calcium acetate |
and to examine its properties. |
|
|||||
|
EQUIPMENT and MATERIALS: |
|
|
|
|
|
||
|
Equipment |
|
• Bosshead |
(2) |
Chemicals and Other Materials |
|
||
|
• Beaker, 500 mL |
(1) |
• Rubber stopper, with one hole (1) |
• Calcium acetate |
|
|||
|
• Test tube, |
(2) |
• Rubber stopper, with two holes (1) |
• Potassium carbonate |
|
|||
|
• Right-angled tube |
(2) |
• Rubber tubing, 50 cm |
(2) |
• Sodium chloride |
|
||
|
• Glass tube, right-angled |
(2) |
• Burner |
(1) |
• Ice |
|
|
|
|
• Support base |
(1) |
• Spatula |
(1) |
• Distilled water |
|
||
|
• Support rod |
(1) |
• Protective glasses |
(1) |
|
|
|
|
|
• Universal clamp |
(2) |
|
|
|
|
|
|
PRE-LAB DISCUSSION
Ketones, like aldehydes, contain the carbonyl group,
In fact a ketone may be regarded as an aldehyde in which the hydrogen in the aldehyde group is replaced by a hydro-
carbon group. Ketones are often prepared by the oxidation of secondary alcohols.
Dimethyl ketone, CH3COCH3, commonly called acetone, is
the simplest and the most important ketone. Acetone is a colourless liquid, boils at 56,5°C and possesses a characteristic pungent odour and sweet taste.
PROCEDURE
Set-up
—Set up the the stand as shown in the Figure with two bossheads and two universal clamps.
—Put 3 spatulas of calcium acetate in a test tube and fix the test tube horizontally on the stand.
Note: Knock the test tube to pack calcium carbonate somewhat down on the bottom of the test tube.
—Fill the half of the beaker with crushed ice and add 6 spatulas of sodium chloride salt.
—Assemble the right-angled tube and the test tubes as seen in the Figure.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
Note: The tip of the right-angled tube must be about 2 cm above the bottom of the test tube in the ice bath.
Note: Be sure that the two test tubes are well closed but there is no tension in the connection.
—Connect a length of rubber tubing to the opening of the right-angled tube so that the end of it should hang over the edge of the laboratory bench.
Note: If possible, down to the floor.
— Wear protective glasses.
Figure
Experiment – 70 How is acetone produced technically?
180

Procedure:
1.Ignite the burner and first heat the whole length of the test tube, then heat the calcium acetate strongly. Record your observations in “Observations and Data Tables”.
Caution : Do not bring the burner near the mouths of the test tubes. Highly inflammable gases are evolved during experiment.
2.Extinguish the burner as soon as about 5 mL of condensate have been collected in the condenser tube. Examine the condensate and carefully test the smell of
the condensate. Record your observations in “Observations and Data Tables”.
3.Add same amount of distilled water into the test tube.
4.Add potassium carbonate onto the mixture in the test tube until the solution is saturated.
5.Close the mouth of the test with a rubber stopper and shake it vigorously. Record your observations in “Observations and Data Tables”.
Note : Air the room after experiment.
OBSERVATIONS AND DATA TABLES 


1.Note your observations you made;
on heating the calcium carbonate : ...................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on examining the condensate : .............................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
on addition of potassium carbonate : ....................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Draw a conclusion from your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Write the reaction equation for the reaction which has taken place.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Write the observed properties of the acetone and look up the missing properties in your text book.
Chemical symbol: ...................... |
Colour : ...................... |
Melting point : ...................... |
Boiling point : ...................... |
Other properties : ............................................................................................................................................................... |
|
|
|
Usage : ............................................................................................................................................................................... |
|
|
|
4.How can alkanones be distinguished from alkanals? Explain
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.What are the usages of the ketones especially acetone in industry? Research.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 70 How is acetone produced technically? |
181 |
|
How can formic acid be prepared?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare formic acid in the laboratory by decomposing oxalic acid.
EQUIPMENT and MATERIALS:
Equipment |
|
• Rubber tubing, 50 cm |
(2) |
• Protective glasses |
(1) |
• Distillation flask, 500 mL |
(1) |
• Support base |
(2) |
|
|
• Crystallising dish |
(1) |
• Support rod |
(2) |
Chemicals and Other Materials |
|
• Condenser |
(1) |
• Universal clamp |
(2) |
• Oxalic acid |
|
• Erlenmeyer flask, 100 mL |
(1) |
• Bosshead |
(2) |
• Glycerol |
|
• Graduated cylinder, 10 mL |
(1) |
• Tripod |
(1) |
• Table salt |
|
• Dropper |
(1) |
• Wire gauze |
(1) |
• Ice |
|
• Thermometer |
(1) |
• Burner |
(1) |
• Boiling chips |
|
• Rubber stopper, with a hole |
(3) |
• Spatula |
(1) |
• Litmus paper, blue |
|
PRE-LAB DISCUSSION
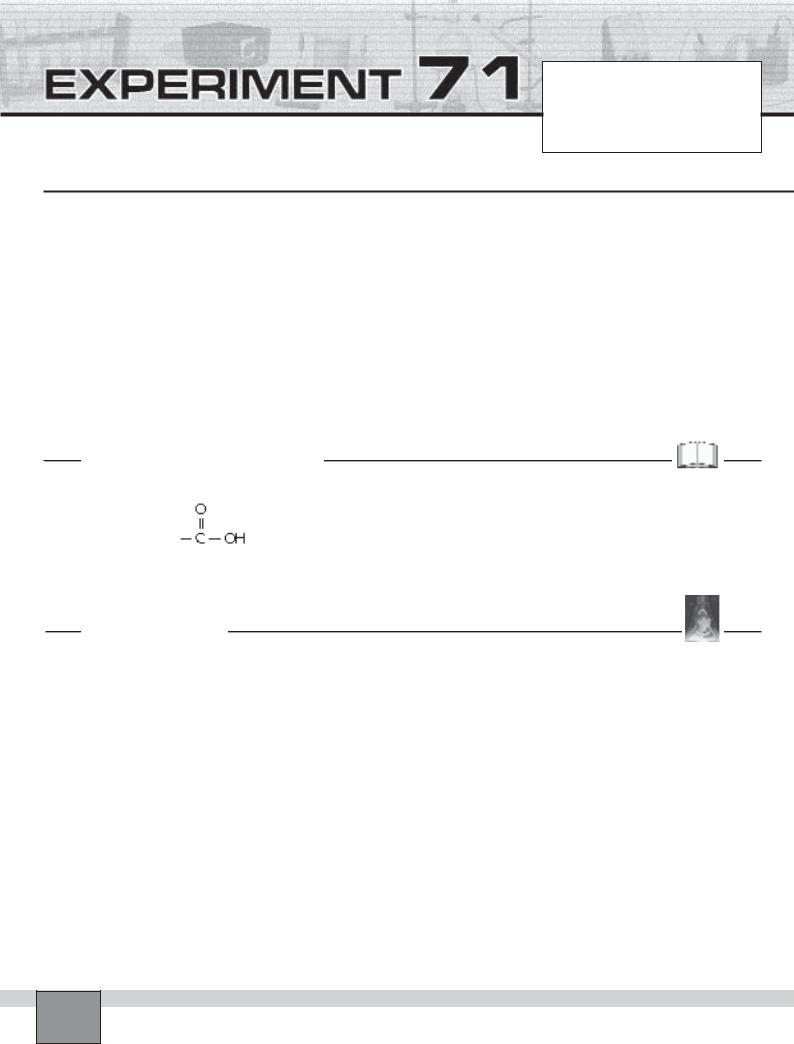
Carboxylic acids contain the carboxyl group,
They are usually weak acids, but they readily form metal salts. Carboxylic acids may be prepared by the oxidation of
primary alcohols or aldehydes.
The simplest carboxylic acid is formic acid, known since 1670. It was isolated by the distillation of red ants. It is partially responsible for the irritation of ant bites and bee stings. Formic acid is a colourless liquid with a sharp, irritating odour.
PROCEDURE
Set-up
— Insert the thermometer into bore of the rubber stopper.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connection.
—Put the distillation flask on the tripod and fix it on the support rod with a universal clamp as in the Figure.
—Weigh 7,5 g of oxalic acid and measure 10 mL of anhydrous glycerol with graduated cylinder then put them into the distillation flask and add some pieces of porcelain.
Caution : Oxalic acid is harmful to health. Do not swallow or let it to contact your skin.
—Close the mouth of the distillation flask with the stopper connected with thermometer.
—Connect the tube of the distillation flask with the condenser with the help of rubber stopper as seen in the Figure.
—Fix the condenser on the second support rod so that it
is held at slight angle.
—Fill the two third of the crystallising dish with crushed ice and add 10 spatulas of table salt.
—Attach the outlet of the condenser with the help of a rubber the erlenmeyer flask which is in the ice bath as seen in the Figure.
—Connect lower side tube of the condenser to the tap and upper one to drain.
—Wear protective glasses.
Procedure
1.Open the tap water and let the water pass through condenser.
2.Ignite the burner and heat the solution gently in the distillation flask until the temperature reach 100°C.
3.When the reaction has taken place for five minutes extinguish the burner and let the distillation flask to cool to about 70°C. Record your observations in “Observations and Data Tables”.
Experiment – 71 How can formic acid be prepared?
182
