
experment book
.pdf
—Sink the closed end of the tube into the water in the beaker and set the apparatus as seen in the Figure.
Note: Hold the bulb of the thermometer about one cm height from the bottom of the beaker. Otherwise it may exploit.
Procedure
1.Measure the height of the trapped air and read the temperature of the water. Record them in the Table in “Observations and Data Tables”.
Note: Wait for a few minutes to allow the temperatures of the water and the trapped air be equal.
2.Heat the water with burner and read measure the height of the trapped air corresponding to the four different temperatures. Record the measurements in the Table in “Observations and Data Tables”.
Caution: Air the room after the experiment.
OBSERVATIONS AND DATA TABLES
1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with your measurements.
Measurements |
|
1 |
|
|
2 |
|
|
3 |
|
|
|
4 |
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Height of trapped air |
|
cm |
|
cm |
|
|
cm |
|
|
cm |
|
cm |
|||||
|
|
|
|
.................. |
|
.................. |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Temperature |
|
.................. oC |
|
.................. oC |
|
.................. |
oC |
|
.................. |
oC |
|
.................. oC |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Temperature |
|
................. oK |
|
|
................. oK |
|
|
.................. |
oK |
|
|
.................. |
oK |
|
|
................. oK |
|
CALCULATIONS
1.Plot the graph of volume versus temperature in Celsius and Kelvin by using experimental values ( Since height and volume are directly proportional, height of the trapped air can be used as volume.).
Experiment – 35 How does temperature affect the volume of a gas? (Charles’s Law) |
93 |
|

2.Calculate the slope of the curve in the Graph-B.
V ...................
Slope = ——— = ——————— = ...................
T ...................
3.Calculate the absolute zero where the volume of gas will be zero, by using Graph-B.
Absolute zero = .................... |
°K = .................... |
°C |
EVALUATIONS AND CONCLUSIONS 


1.Was Charles law verified by this experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Which variables are kept constant for this experiment? Explain why?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Based on the experiment, explain why Kelvin scale is used in the gas behaviour instead of Celsius scale?
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 35 How does temperature affect the volume of a gas? (Charles’s Law)
94

How does temperature affect the pressure of a gas? (Gay-Lussac’s Law)
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the relation between temperature and pressure of a gas.
EQUIPMENT and MATERIALS:
Equipment |
|
• Support rod |
(1) |
• Rubber stopper, with two holes |
(1) |
• Round bottom flask |
(1) |
• Universal clamp |
(1) |
• Protective glasses |
(1) |
• Right angled glass tube |
(1) |
• Bosshead |
(1) |
Chemicals and Other Materials |
|
• Thermometer |
(1) |
• Tripod |
(1) |
• Mercury |
|
• Rubber tubing, 20 cm |
(1) |
• Wire gauze |
(1) |
• Ruler |
|
• Manometer |
(1) |
• Burner |
(1) |
|
|
• Support base |
(2) |
• Test tube holder |
(1) |
|
|
PRE-LAB DISCUSSION
The pressure of a given amount of a gas is directly proportional to the absolute temperature at constant volume. This rule is known as Gay-Lussac’s Law. When the temperature of gas molecules is increased, their kinetic energy, that is velocity, increases. Therefore, the exerted force by gas molecules on the walls of the container increases.
In order to determine the relation between temperature and pressure, volume and quantity of gas must be kept constant.
PROCEDURE
Set-up
—Insert the thermometer into one of the rubber stopper’s holes and the right-angled glass tube into the other hole.
Note: Twist them in cautiously without force and lubricate glassrubber connections with glycerol before connections..
—Clamp the manometer on the support rod.
—Connect the right-angled glass tube with rubber tubing to the manometer.
—Fill the half of the manometer with mercury by using mercury dropper.
Caution: Do not swallow and touch mercury or inhale the mercury vapour because it is extremely harmful. Do this experiment under the hood.
—Set the apparatus as seen in the Figure.
—Wear protective glasses.
—Ask your instructor for the room pressure and record it in the Table in “Observations and Data Tables”.
Procedure
1.Read the temperature of the air in the round bottom flask and measure the difference between the levels of mercury columns. Record them in the Table in “Observations and Data Tables”.
Note: Hold the bulb of the thermometer in the middle of the round bottom flask.
2.Heat the round bottom flask gently and measure the difference between the levels of mercury columns corresponding to four different temperatures.
3.Record the measurements in the Table in “Observations and Data Tables”.
Caution: Air the room after the experiment.
Experiment – 36 How does temperature affect the pressure of a gas? (Gay-Lussac’s Law) |
95 |
|

Figure
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table below with your measurements.
Measurements |
|
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Difference between |
|
|
|
|
|
|
|
|
|
|
mercury levels (hHg) |
|
.............. cm |
|
.............. cm |
|
.............. cm |
|
.............. cm |
|
.............. cm |
|
|
|
|
|
|
|
|
|
|
|
Temperature |
|
oC |
|
oC |
|
oC |
|
oC |
|
oC |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Temperature |
|
K |
|
K |
|
K |
|
K |
|
K |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
Room pressure |
|
|
|
cmHg |
|
|
|
|
|
|
|
|
................ |
|
|
|
|
|
|
|
Experiment – 36 How does temperature affect the pressure of a gas? (Gay-Lussac’s Law)
96

CALCULATIONS
1.Calculate the Pressure of the gas in the round bottom flask by using the formula below.
PGas = PRoom + hHg
Measurements |
|
|
|
2 |
|
|
|
4 |
|
|
|
1 |
|
|
3 |
|
|
5 |
|||
|
|
|
|
|
|
|
|
|
|
|
Pressure of the gas (PGas) |
|
........... cmHg |
|
........... cmHg |
|
........... cmHg |
|
........... cmHg |
|
........... cmHg |
2.Plot the graph of pressure versus temperature in Celsius and Kelvin by using experimental values.
EVALUATIONS AND CONCLUSIONS 


1.Was Gay Lussac law verified by this experiment? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Which variables are kept constant for this experiment? Explain why.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Based on the Graph-B, can the absolute temperature be determined? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 36 How does temperature affect the pressure of a gas? (Gay-Lussac’s Law) |
97 |
|
How can a solution of a substance with desired molarity be prepared?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To prepare a solution of substances with desired molarity by using stock solution and their salts.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Volumetric flask, 50-100 mL |
(1) |
• Sodium Chloride |
• Graduated cylinder, 10 mL |
(1) |
• Sodium hydroxide |
• Calculator |
(1) |
• Hydrochloric acid, concentrated |
• Coloured bottle, 1 L |
(2) |
• Distilled water |
• Wash bottle |
(1) |
|
• Balance |
(1) |
|
PRE-LAB DISCUSSION
Most of the chemicals in laboratory are found as solid or concentrated. But, during experiment, solutions of the chemicals are usually needed. Therefore, solutions which
are desired concentration, should be prepared from stock chemicals. In this experiment, you will prepare salt, acid and base solutions from their stocks in laboratory.
PROCEDURE
Set-up
—Decide and write the volume and the molarity of the substances in the table below.
Note: Choose the molarity between the range 0.1-0.3M and the volume 50 or 100 mL.
Substance |
Concentration |
Volume (V |
2 |
) |
|
|
|
||||
HCl |
.......................M |
....................... |
|
mL |
|
NaOH |
.......................M |
....................... |
|
mL |
|
NaCl ....................... |
M |
....................... |
|
mL |
|
—Calculate the mass of the compounds by using the formulas below
n |
m |
M = —— n = M x V |
and n = ——— m = n x M |
V |
MW |
—Calculate the volume of the needed stock solution of hydrochloric acid by using the formulas below.
d x % |
|
|
|
and M1 x V1 = M2 x V2 |
|||
M = ———— x 10 |
|||
MW |
|
|
|
Procedure
1.Weigh the calculated amount of sodium chloride salt.
2.Place the solid sodium chloride into the 50 mL or 100 mL volumetric flask, then place some distilled water
into the flask as in the Figure.
Note: Graduated cylinder can be used instead of volumetric flask.
3.Shake the volumetric flask slowly to dissolve the salt.
4.Fill the volumetric flask with distilled water until the mark on the flask.
5.Close the tap of the flask and hold the flask with your hand by pressing on the tap with your thumb. Then turn it upside down to mix the solution for several times. Note your observations in “Observations and Data Tables”.
Experiment – 37 How can a solution of a substance with desired molarity be prepared?
98

6. Repeat the above procedure for the sodium hydroxide. |
Figure |
Caution: Handle sodium hydroxide carefully, do not let it touch your skin.
7.Place the calculated amount of concentrated hydrochloric acid in the graduated cylinder.
8.Place some amount of distilled water in the volumetric flask, then add the concentrated acid solution gently.
Caution: Do not add water on concentrated acid solutions, it may splash.
9.Fill the volumetric flask with distilled water until the mark on the flask.
10.Close the tap of the flask and hold the flask with your hand by pressing on the tap with your thumb, then turn it upside down to mix the solution for several times. Note your observations in “Observations and Data Tables”.
11.Store the prepared solution in coloured bottle, and label them.
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
EVALUATIONS AND CONCLUSIONS 


1.Give a procedure for the preparation of a concentrated solution from dilute solution.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Why stock solutions are kept in coloured bottles? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 37 How can a solution of a substance with desired molarity be prepared? |
99 |
|

How can solubility of a solid in water be determined?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To determine the solubility of a solid at different temperatures.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Test tube |
(4) |
• Potassium nitrate |
• Graduated cylinder, 10 mL |
(1) |
• Potassium chlorate |
• Test tube rack |
(1) |
• Potassium chloride |
• Test tube holder |
(1) |
• Sodium nitrate |
• Thermometer |
(1) |
• Distilled water |
• Burner |
(1) |
|
PRE-LAB DISCUSSION
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a constant temperature. Solubility is expressed in grams of solute in 100 g of solvent generally. The amount of solvent is sometimes fixed at 1000 g. Solubility of different substances in a solvent are usually dif-
ferent. Therefore, solubility can be used as characteristic property in a solvent for a substance. Most common solvent is water. Thus, determination of solubility of substances in water is very important.
PROCEDURE
Set-up |
Figure |
—Place four test tubes in the test tube rack.
—Weigh the mentioned amount of salts below,
KNO3 |
: 14 g |
NaNO3 |
: 13 g |
KCl |
: 5 g |
KClO3 |
: 3 g |
—Place them into the test tubes separately and label them.
—Add 10 mL distilled water in the each test tube with graduated cylinder and shake them. Note your observation for each test tube in “Observations and Data Tables”.
Procedure
1.Pick up the test tube containing potassium nitrate with the test tube holder.
2.Heat the contents of the test tube over a small burner flame until the all salt dissolves.
3.Stop heating and allow the solution to cool by stirring continuously with thermometer.
Experiment – 38 How can solubility of a solid in water be determined?
100

4.Observe the formation of the first crystal and read the temperature at which crystal first appear. Record the temperature in the Table in “Observations and Data Tables”.
Note: It is important to stir well to avoid the possibility of supersaturation.
5.Add 10 mL distilled water into the test tube and repeat the steps from 2 to 4.
6.Repeat step 5 for two times by adding 5 mL distilled water.
7.Repeat the steps from 1 to 6 for the other salts and record your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Fill the table with your experimental measurements.
Measurements |
|
KNO3 |
|
|
NaNO3 |
|
|
KCl |
|
KClO3 |
|
Volume of water (Vwater) |
1 |
|
.................. |
oC .................. |
oC |
|
.................. oC |
|
.................. oC |
|
................ mL |
||
2 |
|
.................. |
oC .................. |
oC |
|
.................. oC |
|
.................. oC |
|
................ mL |
||
3 |
|
.................. |
oC .................. |
oC |
|
.................. oC |
|
.................. oC |
|
................ mL |
||
4 |
|
.................. |
oC .................. |
oC |
|
.................. oC |
|
.................. oC |
|
................ mL |
||
CALCULATIONS
1.Calculate the solubility of the salts at four different temperatures and fill the table.(Since density of water is 1, volume of water equals to mass of water in grams.)
msalt
Solubility = ———— x 100
mwater
|
|
KNO3 |
|
|
NaNO3 |
|
|
|
KCl |
|
|
|
KClO3 |
|
|
|
Solubility |
|
|
|
Solubility |
|
|
|
Solubility |
|
|
|
Solubility |
Temp (g/100 g water) |
|
Temp (g/100 g water) |
|
Temp (g/100 g water) |
|
Temp (g/100 g water) |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
||||||||
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
||||||||
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
||||||||
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
|
...... oC ........................ |
||||||||
Experiment – 38 How can solubility of a solid in water be determined? |
101 |
|
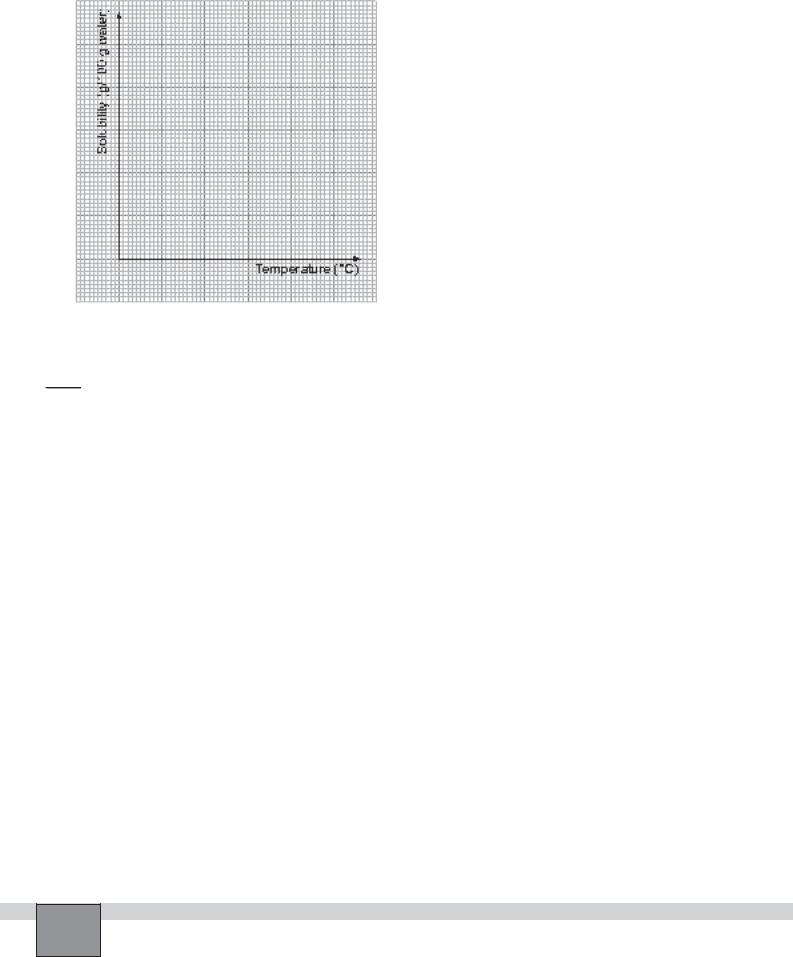
2.Plot the graph of solubility of the salts against the temperature by using the above experimental values.
EVALUATIONS AND CONCLUSIONS 


1.Draw conclusions from your observations and calculations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Does the solubility of any salt in water increase with increase in temperature? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
3.Can solubility of a substance in water be a distinctive property? Explain.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 38 How can solubility of a solid in water be determined?
102
