experment book
.pdf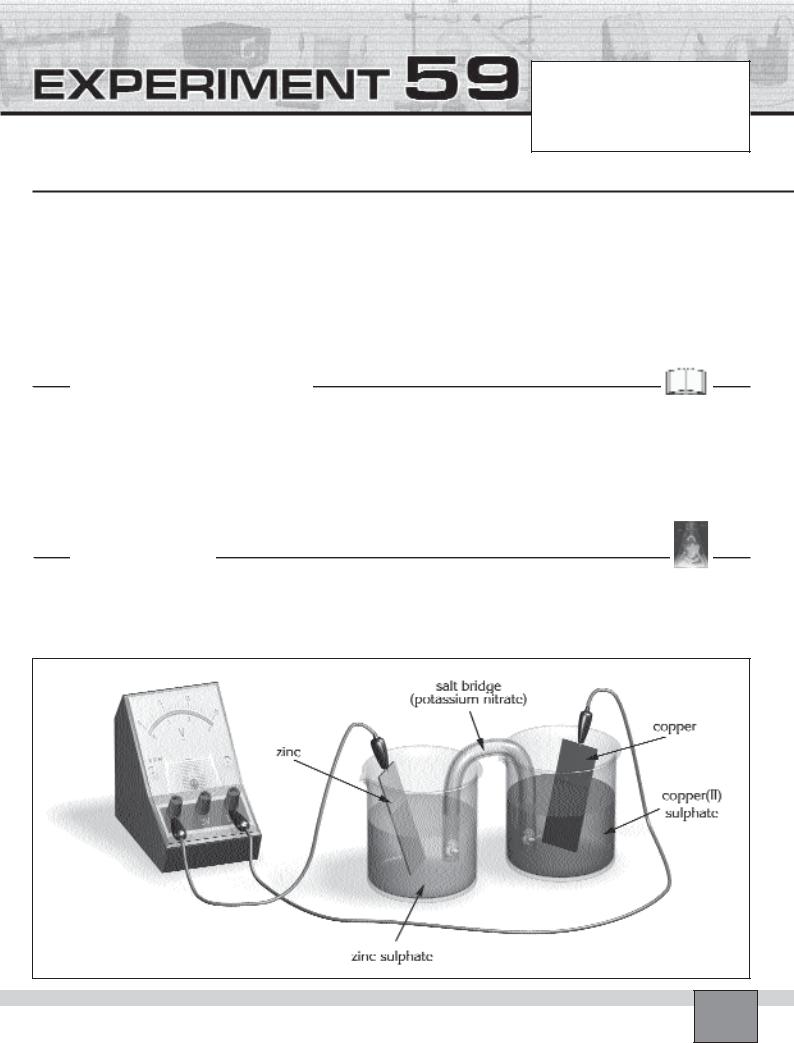
Can a chemical reaction produce electricity?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To establish two half-cells and measure the voltage existing between them.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
|
• Beaker, 250 mL |
(2) |
• Zinc electrode |
• Potassium nitrate solution, 1 M |
• U - tube |
(1) |
• Copper electrode |
• Emery paper |
• DC milli voltmetre |
(1) |
• Zinc sulphate solution, 1M |
• Cotton |
• Crocodile leads |
(2) |
• Copper(II) sulphate solution, 1M |
|
PRE-LAB DISCUSSION
In many redox reactions, there is a complete transfer of electrons from the substance (being oxidised) to the substance (being reduced). If the electrons can be made to travel through an external conductor, during this transfer an electric current will be established in the conductor. In electrochemical cells, the two half reactions, oxidation and reduction reactions, are carried out in separate vessels, called
half cells. The two half cells are connected externally by metal wire attached to the two electrodes. In order to have a complete electrical circuit, electrons must be free to flow from one half cell to the other. This is made possible by connecting the solutions in the two half cells with a salt bridge. The complete system is called an electrochemical cell or simply a chemical cell.
PROCEDURE
Set up
—Clean the Cu and Zn electrodes with emery paper to get shinning surface.
Figure
—Fill a 250 mL beaker half full with 1M copper(II) sulphate solution and immerse Cu electrode in the solution.
Experiment – 59 Can a chemical reaction produce electricity? |
153 |
|
—Fill another 250 mL beaker half full with 1M zinc sulphate solution and immerse Zn electrode into the solution.
—Fill U-tube with 1M potassium nitrate solution and close the openings with cotton lumps.
—Invert the U-tube and place the openings of it in the beakers as shown in the Figure.
—Connect the electrodes, voltmetre and switch with crocodile leads.
Procedure
1.Close the circuit by switching on.
Note: If the needle of voltmetre is deflected in the wrong direction reverse the connections.
2.Read and record the voltage immediately in “Observations and Data Tables”.
3.Wait for 10 minutes and switch off the circuit.
4.Disconnect the electrodes and examine the surface of the electrodes. Note your observations in “Observations and Data Tables”.
OBSERVATIONS AND DATA TABLES 


1.Note your observations.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
2.Record your observations in the table.
Read voltage |
|
Appearance of copper electrode |
|
Appearance of zinc electrode |
|
|
|
|
|
|
|
|
|
|
......................... |
|
.................................................. |
|
.................................................. |
EVALUATIONS AND CONCLUSIONS
1.Indicate the anode and the cathode of the cell.
Anode : ........................................................ Cathode : ........................................................
2. Write the reactions that take place on the surface of the electrodes and overall reaction.
Anode |
: .................................................................................................................................................................... |
Cathode |
: .................................................................................................................................................................... |
Overall |
: .................................................................................................................................................................... |
3.Give a reason for why the process actually takes place and in what direction the electron actually flow.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
...........................................................................................................................................................................................
4.Compare the theoretical and the experimental values and explain the difference.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
5.Explain the role of the salt bridge in the cell.
...........................................................................................................................................................................................
...........................................................................................................................................................................................
Experiment – 59 Can a chemical reaction produce electricity?
154

Can a metal be coated with another metal by electricity?
Date : ...............................................................
Partners : ...............................................................
...............................................................
Grade : ...............................................................
PURPOSE : To coat iron nail with copper metal by electricity in order to protect from corrosion.
EQUIPMENT and MATERIALS:
Equipment |
|
Chemicals and Other Materials |
• Beaker, 250 mL |
(1) |
• Copper electrode |
• Tweezers |
(1) |
• Iron electrode |
• Power supply, (3-12 V) |
(1) |
• Copper(II) sulphate |
• Crocodile leads |
(1) |
• Sulphuric acid, 3M |
• Petri dish |
(1) |
• Hydrochoric acid, 1M |
• Spatula |
(1) |
• Benzene |
|
|
• Distilled water |
• Emery paper
• Filter paper
PRE-LAB DISCUSSION
Metals are coated with another metals to secure improved appearance or greater resistance to corrosion. This is called electroplating. According to this method, a metal object which serves as the cathode in a salt solution is covered
with another metal coating in the course of an electrolysis. The advantage of the method is that a permanent coating can be obtained.
PROCEDURE
Set-up |
Figure |
|
—Clean the iron and copper electrodes with emery paper until they have continuous shining surface.
—Wet a piece of cotton with benzene, then the electrodes with benzene.
Note: From now on, only touch the electrodes with the tweezers.
—Put the iron electrode in the petri dish and add hydrochloric acid until the electrode is just covered.
—After about one minute, turn the electrode around with the help of tweezers.
Note: Be sure that the surface of the iron electrode is completely treated by hydrochloric acid about one minute.
—Hold the iron electrode with tweezers and put it onto the filter paper and let it to dry .
—Place two spatulas of copper(II) sulphate into the 250 mL beaker. Add 50 mL distilled water and then add 50mL sulphuric acid.
Experiment – 60 Can a metal be coated with another metal by electricity? |
155 |
|