
Matta, Boyd. The quantum theory of atoms in molecules
.pdf
142 6 The ELF Topological Analysis Contribution to Conceptual Chemistry and Phenomenological Models
system analysis of the gradient vector field of another function [16, 17], the electron localization function [18]. These methods collectively form what Malcolm and Popelier call quantum chemical topology (QCT) [19], which can be regarded as an important part of conceptual chemistry (by conceptual chemistry we mean the branch of chemistry concerned with analysis of the concepts used by chemists, with their definitions and underlying models). In this chapter we will introduce the electron localization function (ELF) in a very chemical fashion and show how its topology can be used to revisit di erent phenomenological models of bonding or of molecular geometry.
6.2
Why ELF and What is ELF?
The description provided by chemistry considers a molecule as an assembly of atoms linked by bonds. An atom in a molecule consists of a kernel (the nucleus and the inner shell electrons) and valence electrons gathered in the valence shell. The structure of the kernel and the possible numbers of electrons belonging to the valence shell are given by the position of the element in the periodic table. A molecule usually has fewer electrons than the sum of the populations of the valence shells of its atoms, because some of the valence electrons may be shared in two or more valence shells. Such electrons are said to be bonding electrons whereas the other valence electrons are nonbonding. The arrangement of the electrons in the valence shells constitutes the chemical electronic structure. In this description the bonding arises from shared electrons.
One of the objectives of Lewis’s theory of valence [20, 21] is to predict the most probable structure with the help of additional rules such as the octet rule and the rule of two. Lewis’s approach emphasizes the electron pair as a key concept. It is worth noting that an N-electron system has at most N=2 electron pairs in the chemical description and NðN 1Þ=2 in the quantum mechanical description. The chemical approach is not appropriate because many concepts lack a clear definition and because it has no mathematical model behind it.
To recover the Lewis picture within the QCT framework, it is assumed there is a local function, hereafter called the localization function, whose gradient field enables the partitioning of the molecular space into adjacent non-overlapping volumes hereafter called basins in the following way:
1.The space occupied by an atom (with Z > 2) is divided into an inner region, the core basin, encompassing the nucleus, and an external region, the atomic valence shell, gathering its valence basins which may extend to infinity.
2.A valence basin may be shared by the valence shells of several atoms.
3.There is a high probability of finding Z nv electrons within a core basin where nv is the ordinal number of the group of the periodic table to which the element belongs (in other words the conventional number of valence electrons).

6.2 Why ELF and What is ELF? 143
4.There is a high probability of finding an even number of electrons in a valence basin belonging to a closed-shell system.
The number of electrons NðWAÞ in each basin WA is given by the integrated density:
|
|
ðWAÞ ¼ ð |
rðrÞ dr |
ð1Þ |
N |
||||
|
|
|
WA |
|
which is, alternatively, the expectation value of the basin population operator NðWAÞ [22], i.e.:
^ |
ð2Þ |
NðWAÞ ¼ hCjNðWAÞjCi |
NðWAÞ is, therefore, a statistical average, the variance of which provides a measure of the electron localization. Zero variance corresponds to perfect localization within WA whereas a large variance is an indication of a poor localization. The variance localization criterion can be generalized to any volume and, in particular, to a sampling volume v around a reference point of coordinate r. The variance s2ðrÞ is expressed as:
s2ðrÞ ¼ |
N |
?ðrÞ þ |
N |
==ðrÞ |
N |
2ðrÞ þ |
N |
ðrÞ |
ð3Þ |
in terms of the population NðrÞ, and of the opposite spin and the same spin pair populations:
|
|
?ðrÞ ¼ ðvðpab ðr1; r21Þ þ pab ðr1; r21ÞÞ dr1 dr21 |
|
N |
ð4Þ |
||
|
|
==ðrÞ ¼ ðvðpaaðr1; r21Þ þ pbb ðr1; r21ÞÞ dr1 dr21 |
|
N |
ð5Þ |
respectively. To check the ability of s2ðrÞ to locate the basin boundaries, let us consider the very simple model of four electrons distributed in two identical cubic boxes, A and B, of volume V0, sharing a face, we further impose the condition there is always an opposite spin pair in each box and that the electron density is constant within the boxes. In this circumstance the electron density and the pair functions have the expressions:
rðrÞ ¼ |
2:0 |
¼ r for r A A or B |
ð6Þ |
||||||
|
|
|
|||||||
|
V0 |
||||||||
pss r; r0 |
|
|
81:0 |
for r or r0 |
in the same box |
7 |
|||
|
|
|
Þ ¼ < |
0:0 |
|
|
|||
ð |
|
|
|
for r and r0 in di erent boxes |
ð Þ |
||||
|
|
2 |
|||||||
|
|
|
|
|
:V0 |
|
|
|
|

144 6 The ELF Topological Analysis Contribution to Conceptual Chemistry and Phenomenological Models
p |
ss 0 |
ðr; r0Þ ¼ |
1:0 |
|
|
|
for r and r0 A A or B |
ð8Þ |
||||||||||||||
|
|
|
|
V 2 |
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
||
which imply that both ‘r and ‘2r are identically zero and that: |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ð9Þ |
|||||
|
N |
ðrÞ ¼ rv |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
and |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
1 |
|
|
|
|
|
1 |
|
|
|
|
|
||||||
N?ðrÞ ¼ |
|
|
r |
2v2 |
¼ |
|
|
N2ðrÞ |
ð10Þ |
|||||||||||||
2 |
2 |
|||||||||||||||||||||
and thus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
s2ðrÞ ¼ NðrÞ |
N2ðrÞ þ N==ðrÞ |
ð11Þ |
||||||||||||||||||||
|
||||||||||||||||||||||
2 |
||||||||||||||||||||||
If v is chosen to constrain NðrÞ to be constant, the only varying quantity in s2ðrÞ is N==ðrÞ, which is zero if the sampling volume is totally in one box and which is maximum when it is equally shared by the two boxes, that is when the reference point belongs to the boundary. The integrated same spin pair density over a sampling volume around a reference point is thus a convenient descriptor of pair formation in the sense of Lewis’s model. The spin pair composition [23] defined as:
cpðrÞ ¼ |
|
ðrÞ 8=3 |
|
==ðrÞ |
ð12Þ |
N |
N |
is a quantity independent of NðrÞ for small NðrÞ values.
It has been shown that ELF [18] is an excellent approximation to this function when put in the Lorentzian form hðrÞ ¼ ð1 þ cp2ðrÞÞ 1 which confines the values of hðrÞ in the [1, 0] interval. ELF was originally conceived as a measure of the Fermi hole curvature [18, 24] of an Hartree–Fock wavefunction. It has the advantage that it can be expressed analytically in terms of basis functions in all practical cases where the wavefunction is expressed in terms of orbitals whereas the spin pair composition must be calculated numerically. ELF has been alternatively interpreted in terms of local excess kinetic energy because of Pauli repulsion [25], in terms of localized orbitals [26], and, recently, as the nonadditive (inter-orbital) Fisher information contained in the electron distribution [27].
6.3
Concepts from the ELF Topology
The ELF topology has been extensively used for study of chemical bonding [28– 80], aromaticity problems [81–88], reactivity [89–91], and chemical reactions [92– 105]. Several review papers have already been published [86, 106–108]. The ELF

6.3 Concepts from the ELF Topology 145
theory provides very appealing pictures of bonding and a very ‘‘chemical’’ population analysis.
6.3.1
The Synaptic Order
Topological partitioning of the ELF gradient field [16, 17] yields basins of attractors which can be thought of as corresponding to atomic cores, bonds, and lone pairs. In a molecule one can find two types of basin:
1.core basins surrounding nuclei with atomic number Z > 2 and labeled C(A), where A is the atomic symbol of the element, and
2.the valence basins.
The valence basins are characterized by the number of atomic valence shells in which they participate. This number is called the synaptic order [109]. There are, therefore, monosynaptic, disynaptic, trisynaptic basins, etc. Monosynaptic basins, labeled V(A), correspond to the lone pairs of the Lewis model, and polysynaptic basins to the shared pairs of the Lewis model. In particular, disynaptic basins, labeled VðA; XÞ, correspond to two-center bonds, trisynaptic basins, labeled VðA; X; YÞ, to three-center bonds, etc. The valence shell of a molecule is the union of its valence basins. Because hydrogen nuclei are located within the valence shells of at least two atoms they are located in the corresponding polysynaptic basin. For example, the valence basin accounting for a CaH bond labeled VðC; HÞ contains a proton and is called, for this reason, protonated disynaptic. The valence shell of an atom, say A, in a molecule is the union of the valence basins whose label lists contain the element symbol A. Figure 6.1 shows two examples of trisynaptic basins – the protonated trisynaptic basins of diborane VðB; B; HÞ, which provides a picture close to the ‘‘protonated double bond’’ of Pitzer [110], and the pentacoordinated carbons of Al2C2H10, which involves three VðC; HÞ disynaptic basins and one VðAl; Al; CÞ trisynaptic basin.
6.3.2
The Localization Domains
In the context of ELF analysis the concept of a domain is very important, because it enables definition of the chemical units within a system and characterization of
Fig. 6.1 Localization domains of B2H6 (left) and Al2C2H10 (right).

146 6 The ELF Topological Analysis Contribution to Conceptual Chemistry and Phenomenological Models
the valence domains belonging to a given chemical unit. The mathematical properties of the gradient dynamical system do not, by themselves, provide the whole set of definitions necessary to describe the bonding in molecules. Other mathematically based approaches are therefore required for this purpose.
The topological concept of domain was introduced to chemistry by P. Mezey to recognize functional groups within organic molecules [111]. Generalized to ELF isovalues this concept has proven to be an e cient ‘‘generator’’ of clear definitions. Any subset of molecular space bounded by an external closed isosurface hðrÞ ¼ f is a domain. An f-localization domain is such a subset, with the restriction that each point satisfies hðrÞ > f . A localization domain which surrounds at least one attractor is called irreducible. If a localization domain contains more than one attractor it is reducible. An irreducible domain is a subset of a basin whereas a reducible domain is the union of subsets of di erent basins. Except for atoms and linear molecules, the irreducible domains are always filled volumes whereas the reducible domains can be either filled or hollowed volumes. If the value of hðrÞ defining the bounding isosurface is increased a reducible domain splits into several domains each containing fewer attractors than the parent domain. Reduction of localization occurs at turning points, which are critical points of index 1 located on the separatrix of two basins involved in the parent domain. Ordering these turning points (localization nodes) by increasing hðrÞ enables one to build tree-diagrams reflecting the hierarchy of the basins.
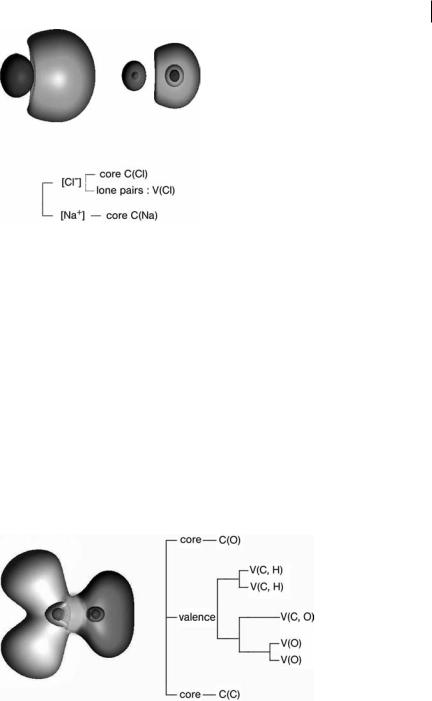
For any system there are low values of hðrÞ ¼ f defining a unique composite parent domain. The successive reductions of localization will split this parent domain. Every branch which is a composite domain corresponds to one or more chemical species. A chemical unit is the union of the basins of the last appearing composite domain of a branch, if it is a filled volume. In a complex such as the weak hydrogen bonded system FH ClH shown in Fig. 6.2 the first reduction yields two composite domains corresponding to the interacting moieties. Such a complex cannot be regarded as being chemically bonded [39]. In the same way, in an ionic pair such as NaCl, the first reduction yields domains corresponding to the cation and to the anion as shown in Fig. 6.3. In a molecule the initial parent domain first splits into core domains and a single valence domain which contains
Fig. 6.2 h(r) ¼ 0:06 localization domains and reduction of the localization tree-diagram of FH ClH.
6.3 Concepts from the ELF Topology 147
Fig. 6.3 h(r) ¼ 0:05 (left) and h(r) ¼ 0:5 (right) localization domains and reduction of the localization tree-diagram of NaCl.
all the valence attractors. The shape of this latter domain is that of a hollowed volume with as many holes as atomic cores in the molecule. Each hole contains a core domain as displayed for H2CO in Fig. 6.4.
6.3.3
ELF Population Analysis
ELF population analysis relies on the integrated density over the basin volumes. The basin populations are the expectation values of the population operator introduced by Diner and Claverie [112]:
N^ |
ð |
WA |
N |
^y ri |
Þ |
with ^y |
ri |
Þ ¼ |
1 ri A WA |
N^ |
ð |
WA |
Þ |
13 |
|
|
Þ ¼ Xi |
ð |
ð |
|
0 ri B WA |
|
|
ð Þ |
Fig. 6.4 h(r) ¼ 0:4 localization domains and reduction of the localization tree-diagram of H2CO.

148 6 The ELF Topological Analysis Contribution to Conceptual Chemistry and Phenomenological Models
where N denotes the total number of electrons. The eigenvalues, NðWAÞ, of
^ð Þ ; . . . ;
N WA belong to the series of integer values 0 N and represent all the accessible numbers of electrons within WA. The eigenvalues of the population operators of di erent basins are correlated, because they also obey the closure relationship:
X |
ð14Þ |
NðWAÞ ¼ N |
A
The expectation values of the population operators given by Eq. (2) can be expressed in terms of the volume integral of the one-electron probability distribution over the basins (Eq. 1). They are real numbers and can be understood as the average of measurements of the electron numbers NðWAÞ. They also fulfill a closure relationship, i.e.:
X
|
|
|
|
NðWAÞ ¼ N |
ð15Þ |
||
A
These eigenvalues and expectation values are, in fact, determined simultaneously. Each set of eigenvalues defines an accessible chemical electronic structure and the expectation values NðWAÞ can therefore be interpreted as weighted averages of mesomeric structures. The closure relation of the basin population operators enables one to perform a statistical analysis of the basin populations by definition of a covariance matrix [22]. The covariance operator is a matrix operator whose elements are deduced from the classical expression of the covariance:
^ |
^ |
^ |
|
|
|
|
ð16Þ |
|
|
|
|
||||
covðWA; WBÞ ¼ NðWAÞNðWBÞ NðWAÞNðWBÞ |
|||||||
The covariance matrix element expectation values are the di erences between the actual pair populations PðWA; WBÞ and their ‘‘classical’’ analogs NðWAÞNðWBÞ, or NðWAÞðNðWAÞ 1Þ for the diagonal elements:
hcov^ ðWA; WAÞi ¼ |
P |
ðWA; WAÞ |
N |
ðWAÞð |
N |
ðWAÞ 1Þ |
ð17Þ |
|||||
hcov^ ðWA; WBÞi ¼ |
|
|
|
|
|
|||||||
P |
ðWA; WBÞ |
N |
ðWAÞ |
N |
ðWBÞ |
ð18Þ |
||||||
The diagonal elements of the covariance matrix (the variances), are often denoted s2ðNÞ, because they classically represent the square of the standard deviation s.
For open-shell systems it is also very interesting to localize the unpaired electrons by calculating the integrated spin density over localization basins.
Although the topological representation enables rather satisfactory interpretation of the bonding, reliable descriptions in terms of the superposition of chemical structures are often very helpful, at least as explanatory models. As has been proposed in two previous papers [22, 81], the data provided by the topological
|
|
|
|
6.4 VSEPR Electron Domains and the Volume of ELF Basins |
149 |
||||
Table 6.1 |
Valence basin populations (e) and covariance matrix elements of H2CO. |
|
|
|
|||||
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
Basin |
|
|
|
|
hcov(^ W, WO)i |
|
|
|
|
N(W) |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
V(C, H) |
V(C, O) |
V(O) |
|||
|
|
|
|
|
|
|
|
|
|
VðC; HÞ |
2.13 |
0.52 |
0.17 |
0.07 |
|
||||
VðC; OÞ |
2.43 |
0.17 |
1.16 |
0.40 |
|
||||
V(O) |
2.52 |
0.07 |
0.40 |
0.98 |
|
|
|||
analysis can be used to build such models and to evaluate their ability to describe the distribution of electrons. To illustrate this procedure we consider the formaldehyde molecule as an example. The valence basin populations and their covariances are reported in Table 6.1.
The three mesomeric structures considered are:
(a)H2CbO
(b)H2Cþ aO
(c)H2C Oþ
The weights calculated for these structures are 0.24, 0.58, and 0.18 for (a), (b), and (c), respectively. They yield populations and covariance in good agreement with population analysis.
6.4
VSEPR Electron Domains and the Volume of ELF Basins
The VSEPR model relies on a distribution of the valence electron pairs among bonding and nonbonding electron domains [12, 13] which are defined as the regions of space in which the probability of finding an (opposite spin) electron pair or a large fraction of an electron pair is high. It is assumed that:
1.nonbonding domains or lone pairs are larger in size than bonding domains and are therefore more repelling;
2.the size of the bonding domains decreases when the electronegativity of the ligand increases and/or the electronegativity of the central atom decreases; and
3.multiple bonds have larger domains than a single bonds.
The topology of the Laplacian of the charge density has been invoked as a physical basis for the VSEPR model [113]. Qualitatively, the valence shell charge concentrations (VSCCs) of the central atom correspond to the electronic domains of the model [12, 13] and they have also been used to explain the geometries of non-

150 6 The ELF Topological Analysis Contribution to Conceptual Chemistry and Phenomenological Models
VSEPR molecules [114]. Malcolm and Popelier attempted to justify quantitatively the VSEPR assumptions on the relative domain sizes by considering the full topology of the Laplacian of the charge density [19]. They concluded that nonbonding domains are larger than bonding domains belonging to the same valence shell and that multiple bond domains are larger than single bond domains, but they found that the full topology of the Laplacian does not account for electronegativity e ects.
There is, in principle, a one-to-one correspondence between the VSEPR electronic domains and the valence basins of the ELF function. To define finite volumes, the valence basins are limited by a bounding density isosurface. We have analyzed the volumes of the basins of approximately 150 molecules. Mathematically a basin is allowed to extend to infinity, which is not chemically meaningful; we have therefore limited molecular space by the density isosurface rðrÞ ¼ 10 4 bohr 3, which ensures that no more than 0.2e are omitted.
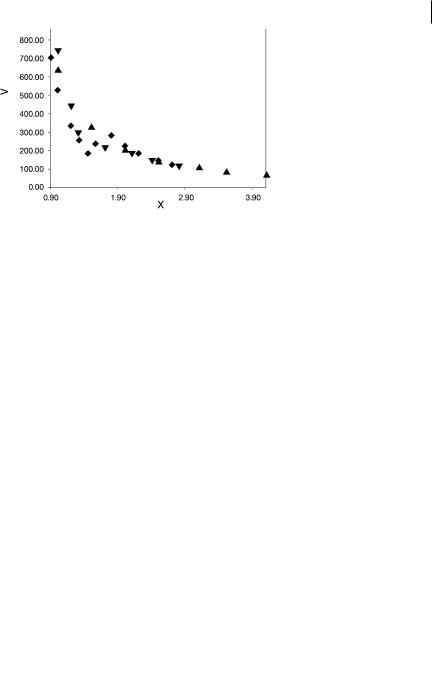
The volumes of the core basins of the main group elements of the four first periods range from 0.1 bohr3 (Ne) to 149.5 bohr3 (K). For the group 13–17, however, the largest value is only 16.5 (Ga). The core volumes of the fourth-period transition metals are in the range 50.5–20.5 bohr3 (Sc and Ni respectively). It is worth noting that in transition-metal molecules the core external shell is split into several basins [66] the volumes of which are of the order of a few bohr3. Figures 6.5 to 6.8 depict the volumes of the V(X) lone-pair basins and the VðX; HÞ and VðX; XÞ bonding basins as functions of the Allred and Rochow electronegativity of X [115]. They reveal very good correlations that are almost period-independent. For a given element the largest deviation occurs for the V(N) basin of CH3CN whose population is 3.2e. As a general rule, basin volumes decrease when the
Fig. 6.5 Dependence of V(X) volume (bohr3) on w(X) for HF, HCl, HBr, H2O, H2S, H2Se, NH3, PH3, AsH3, CH3F, CH3Cl, CH3Br, CH3OH,
CH3NH2, H2CO, H2CNH, F2, Cl2, and Br2. Csecond period elements, Dthird period elements, Yfourth period elements.
6.4 VSEPR Electron Domains and the Volume of ELF Basins 151
Fig. 6.6 Dependence of V(A, H) volume (bohr3) on w(A) in AHn hydrides. Csecond period elements, Dthird period elements, Yfourth period elements.
electronegativity of X increases. The shrinking of the V(X) basin with electronegativity is a consequence of the radial decay of the electron density, which is governed by the core net charge. For the VðX; HÞ and VðC; XÞ bonding basins the observed behavior can be explained by the electronegativity di erences which determine the ionic character of the bond. For wðXÞ > wðHÞ or wðXÞ > wðCÞ the ionic contributions involving either Hþ or CR3þ tend to reduce the basin volume whereas the anionic contribution H or CR3 act in the opposite direction when wðXÞ < wðHÞ or wðXÞ < wðCÞ. Finally, the decrease of the VðX; XÞ basin volume
Fig. 6.7 Dependence of V(A, A) volume (bohr3) on w(A). Csecond period elements, Dthird period elements, Yfourth period elements.
