
- •Preface
- •Acknowledgments
- •Basic Physics
- •Transducers and Resolution
- •Doppler Physics
- •Artifacts
- •Summary
- •Introduction
- •Patient Preparation
- •Patient Positioning
- •Transducer Selection
- •Two-Dimensional Images
- •Two-Dimensional Imaging Controls
- •Introduction
- •M-Mode Echocardiography
- •Color-Flow Doppler
- •Spectral Doppler
- •Tissue Doppler Imaging
- •Measurement and Assessment of Two-Dimensional Images
- •Measurement and Assessment of M-Mode Images
- •Measurement and Assessment of Spectral Doppler Flow
- •Measurement and Assessment of Tissue Doppler Imaging
- •Evaluation of Color-Flow Doppler
- •Evaluation of Ventricular Function
- •Mitral Regurgitation
- •Aortic Regurgitation
- •Tricuspid Regurgitation
- •Pulmonary Regurgitation
- •Endocarditis
- •Pulmonary Hypertension
- •Systemic Hypertension
- •Hypertrophic Cardiomyopathy
- •Dynamic Right Ventricular Outflow Obstruction
- •Moderator Bands
- •Dilated Cardiomyopathy
- •Right Ventricular Cardiomyopathy
- •Restrictive Cardiomyopathy
- •Endocardial Fibroelastosis
- •Arrhythmogenic Right Ventricular Cardiomyopathy
- •Myocardial Infarction
- •Myocardial Contusions
- •Pericardial Effusion
- •Neoplasia as a Cause of Pericardial Effusion
- •Pericardial Disease
- •Abscesses
- •Pericardial Cysts
- •Thrombus
- •Ventricular Septal Defect
- •Patent Ductus Arteriosus
- •Aorticopulmonary Window
- •Right to Left Shunting PDA
- •Atrial Septal Defects
- •Endocardial Cushion Defects
- •Bubble Studies
- •Atrioventricular Valve Dysplasia
- •Outflow Obstructions
- •Inflow Obstructions
- •Tetralogy of Fallot
- •APPENDIX ONE Bovine
- •APPENDIX TWO Canine
- •APPENDIX THREE Equine
- •APPENDIX FOUR Feline
- •APPENDIX FIVE Miscellaneous Species
- •Index

causes hypertrophy of the septum and wall. One study showed free wall hypertrophy in 72% of cats with hyperthyroidism while only 40% of them had septal hypertrophy. Seventy percent of these same cats also had left atrial dilation. Almost 50% of these cats are reported to have an increase in ventricular diastolic chamber size. The vast majority of cats have hyperdynamic wall and septal motion and increased fractional shortenings (71,73,74). While there appears to be some differences between idiopathic and non-idiopathic left ventricular hypertrophy in the cat, there is no reliable M- mode or two-dimensional echocardiographic way to differentiate between these causes of hypertrophy. They may even coexist (10,70,75,76). The degree of hypertrophy, the incidence of SAM, and the degree of outflow obstruction are similar in both hypertensive and idiopathic hypertrophic cardiomyopathy patients in man. The presence of hypertension in conjunction with HCM usually results in a more significant hypertrophy however (77). There are also irreversible causes of left ventricular hypertrophy that can mimic HCM. These include hypertrophic feline muscular dystrophy and other infiltrative disorders (78,79). In man tissue Doppler has been shown to help differentiate between pathologic left ventricular hypertrophy (HCM, hypertension) and the hypertrophy found in athletic hearts. Athletic hearts showed normal systolic and diastolic annular myocardial velocities while they are altered in the pathologic forms (58).
Rule out reversible causes of hypertrophy such as hypertension and hyperthyroidism. They cannot be differentiated from HCM echocardiographically.
An unusual presentation for cats with hyperthyroidism is left and right ventricular chamber dilation with normal wall and septal thickness and poor fractional shortening. This is thought to occur secondary to increased systolic wall stress and myocardial dysfunction with chronic hyperthyroidism leading to congestive heart failure. Even these changes are reversible to a certain degree as the hyperthyroid state is corrected (80).
Dynamic Right Ventricular Outflow Obstruction
Right ventricular outflow tract obstruction is occasionally seen in people and has been reported in cats (10,81–83). The cause of the obstruction is not well defined and may simply be secondary to excessive hypertrophy of the right ventricular wall or septum into the right ventricular outflow tract or cavity. Color-flow Doppler documents the presence of turbulent flow within the right ventricular outflow tract and spectral Doppler displays a late-peaking flow velocity. This is a dynamic and variable obstruction to flow and is termed dynamic right ventricular outflow obstruction (DRVO). Frame-by- frame analysis can show septal and right ventricular free wall apposition at the point where high velocity flow originates. The gradients associated with this RV outflow obstruction are low with velocities ranging from 1.7 to 4.0 m/sec in cats and man (mean and median 2.4 and 2.3 m/sec, respectively) (Figure 7.30) (10,81,82). This is sometimes the only detectable abnormality in some cats with murmurs, and clinical signs related to cardiac disease are not present. The absence of other cardiac disease is more common in older cats than in young cats with DRVO. Reported associations in the cat include HCM, chronic renal failure with and without hypertension, hyperthyroidism, anemia, neoplasia, and inflammatory processes (81,82). Hyperdynamic function especially when associated with some of the listed conditions or with increased sympathetic stimulation are hypothesized as underlying causes of DRVO (82).

Dynamic right ventricular outflow obstruction may be the cause of some murmurs in cats.
Figure 7.30 Dynamic right ventricular outflow obstruction with a late peaking pulmonary flow profile (arrows) occurs in some cats. This may be the only source of a murmur.
Moderator Bands
False tendons or moderator bands may be a normal finding in many animals and are common in the right side of the heart (Figure 7.31). Occasionally they may restrict left ventricular filling. These tendons cross the ventricular cavity connecting the septum and free wall or papillary muscles (84). Echocardiographic abnormalities include a rounded left ventricular apex, irregular contours to the chamber, and a narrowed ventricular chamber. These cats may present with symptoms very similar to cats with cardiomyopathy of any form (85). Not all moderator bands create problems however and the identification of a false tendon by echocardiography should not be immediately associated with cardiac malfunction (84,85,87).
Figure 7.31 (A) Moderator bands are seen within the apex of this cat’s heart (arrow). They created no problems for this animal. (B) A moderator band or aberrant chordae is seen along the ventricular septum (arrow) in this cat. RV = right ventricle, LV = left ventricle, VS = ventricular septum, LVW = left ventricular wall, AO = aorta, LA = left atrium, RA = right atrium, plane = right parasternal left ventricular outflow view, species = feline.

Dilated Cardiomyopathy
Idiopathic dilated cardiomyopathy (DCM) is a myocardial disease of unknown etiology. In man there are some known causes of dilated cardiomyopathy including toxins like ethanol and lead, metabolic abnormalities such as nutritional and endocrine disorders, inflammatory and infectious processes, neuromuscular diseases including several dystrophies, and there is a definite familial component (88– 90). The cause of most dilated cardiomyopathies in animals remains unknown, but several potential underlying causes are being studied. These include carnitine deficiencies, metabolic abnormalities including hypothyroidism, and myocarditis (88–97). Taurine deficiency was established as a cause of dilated cardiomyopathy in cats, and now the disease is rare in the feline (98–101). Plasma taurine levels are not low in dogs with DCM except for possibly the Cocker Spaniel, and were actually found to be significantly higher than levels found in normal healthy dogs (102). Familial dilated cardiomyopathy is reported in Doberman Pinschers, Boxers, English Cocker Spaniels, Irish Wolfhounds, young Portuguese water dogs, Great Danes, and Newfoundlands (103–110). In Portuguese water dogs, the disease strikes young puppies, and the time from clinical presentation to

the development of congestive heart failure is weeks (110). The prominent features of DCM are similar in dogs, cats, and horses and include ventricular dilation, atrial dilation, normal or thin wall and septal thicknesses, depressed systolic thickening of the wall and septum, poor fractional shortening, large E point to septal separation (EPSS), reduced aortic wall motion, and global hypokinesis (11,88,90,100,110–117).
Two-Dimensional and M-Mode Evaluation
Ventricular dilation and poor function are the hallmarks of DCM (Figures 7.32, 7.33). Several breeds display either a lack of dilation or extreme dilation because of the myocardial disease, and these include the Boxer, Doberman Pinscher, and the Dalmatian (118–121). The Dalmatian appears to exhibit more ventricular and atrial dilation than any breed with DCM. Boxer DCM may have normal ventricular chamber size despite poor left ventricular systolic function. Dobermans tend to display less right-sided involvement than other breeds with DCM (115). Left ventricular diastolic dimensions greater than 4.6 cm and systolic left ventricular dimensions greater than 3.8 cm with fractional shortening of <25% are considered to be diagnostic of dilated cardiomyopathy in the Doberman (122). The dilation generally involves the left side of the heart. The right ventricle may or may not be affected (11,117,123). The dysfunction is diffuse and involves the entire ventricle, but still some areas may exhibit better function than others may. This is thought to be secondary to variable areas of abnormal wall stress (88,94,111,123). It is important to remember that fractional shortening alone should not be used as the defining criteria for the diagnosis of DCM (110). Fractional shortening is preload and afterload dependent, and transient depressions in this parameter can occur normally (124). Increased chamber size and increased EPSS combined with poor fractional shortening are usually indicative of the presence of DCM. Serial studies are often necessary before the diagnosis can be made with certainty (110,125).
Dilated Cardiomyopathy
Features
Dilated left ventricle
Some breeds do not show this
Atrial dilation
Normal to thin LVW and VS
Poor fractional shortening
Large EPSS
Figure 7.32 These images display (A) severe, (B) moderate, and (C) mild left ventricular and atrial dilation in dogs with dilated cardiomyopathy. The wall and septum are thin for the volumes in these hearts. RV = right ventricle, LV = left ventricle, RA = right atrium, LA = left atrium, AO = aorta, plane = right parasternal left ventricular outflow view.

Figure 7.33 Left ventricular function is depressed to varying degrees in these animals with dilated cardiomyopathy. (A) a horse showing virtually no contraction of the septum or wall, (B) a cat with
poor function and pleural effusion, (C) some contraction is still evident in the septum of the dog but the free wall is akinetic, (D) the wall and septum still thicken slightly in this dog’s heart, and (E) no visible contractions are present on this M-mode. RV = right ventricle, VS = ventricular septum, LV = left ventricle, LVW = left ventricular wall.


Early DCM may exhibit only increased systolic left ventricular dimensions and normal diastolic dimensions as fractional shortening decreases. Diastolic chamber size will increase as function deteriorates and forward flow is compromised (116,117,126). One study in cats with taurine deficiency showed only increased systolic left ventricular dimensions and decreased fractional shortening with no dilation of the ventricle during diastole (98). Another study however did show left ventricular dilation (100).
Left atrial and sometimes right atrial dilation develop as myocardial failure progresses (100). Elevated left ventricular end systolic pressure creates less of a pressure gradient from the atrium into the ventricle resulting in less atrial emptying, high left atrial pressures, and atrial dilation (11,90,115,116). Some cats display normal left atrial size on echocardiographic examinations but do have parameters that are at the high end of the normal range (11). Echocardiographic evidence of atrial enlargement may be present before there is radiographic indication of atrial dilation. As with hypertrophic cardiomyopathy, a thrombus may form within the left atrial or auricular chamber (Figure 7.34).
Figure 7.34 A clot has formed in the left atrium of this cat with dilated cardiomyopathy. Left
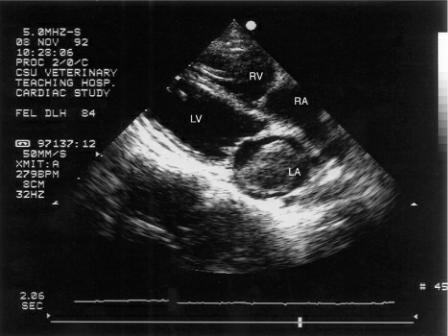
ventricular chamber size was still within the normal range but fractional shortening was very poor. The right side of the heart appears dilated. RV = right ventricle, LV = left ventricle, RA = right atrium, LA = left atrium, plane = right parasternal four chamber.
Septal and free wall thicknesses may be normal but are typically thin (11,100,116,117,125). There is no measurable hypertrophy even though heart size and weight are increased. A decrease in wall thickness to chamber size ratio implies inadequate hypertrophy and myocardial abnormalities. Prognosis is poorer in human patients with low ratios (127,128). The wall and septum also have reduced systolic thickening and excursions as myocardial function deteriorates. The lack of compensatory hypertrophy as the ventricle dilates increases left ventricular afterload and wall stress further decreasing fractional shortening and velocity of circumference shortening. In Irish Wolfhounds with DCM and normal septal thickness, there is reduced systolic thickening (125).
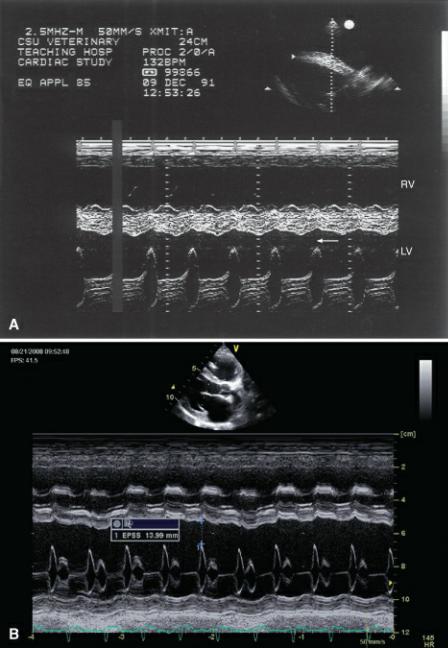
The E point to septal separation (EPSS) is always increased in dogs and cats with DCM (Figures 4.38, 7.35) (100,125). The degree of mitral valve motion toward the interventricular septum is dependent upon flow from the atrium into the ventricle. As end systolic volume remains large secondary to poor contraction, elevated left ventricular filling pressure limits the amount of blood flowing from the atrium into the ventricle. Mitral excursion is reduced as a result and EPSS increases. This is a sensitive and specific sign of early DCM even in asymptomatic dogs and in dogs with equivocal values for all other parameters of size and function (116). The EPSS in Doberman Pinchers shows no overlap between Doberman’s with normal function and those with occult DCM making it an important factor in the assessment of DCM in this breed (116). There is overlap in EPSS measurements between normal Irish Wolfhounds and Irish Wolfhounds with DCM however (125).
Figure 7.35 (A) A large EPSS in this horse is present secondary to poor ventricular function (arrow). The right ventricle is also dilated. (B) Large E point to septal separations correlate inversely with ejection fraction in animals with dilated cardiomyopathy. This dog has a large EPSS (measurement). There is also right ventricular dilation. RV = right ventricle, LV = left ventricle.
A “B” bump at the end of mitral valve diastolic motion signifying delayed closure is a sign of increased end diastolic left ventricular pressures. This may or may not be found in dogs with DCM (Figures 4.99, 5.24) (113,116). It has routinely only been seen in dogs with severe congestive heart failure.
Parameters of systolic function are always altered in DCM. Fractional shortening, ejection time, and Vcf are decreased while pre-ejection period (PEP) is increased (Figure 7.36) (129–131). The ratio of PEP to left ventricular ejection time (LVET) is increased in DCM secondary to slow rate of pressure rise during isovolumic contraction. This value is not sensitive however, as PEP/LVET ratios may still be normal in dogs with overt heart signs of heart failure (116). LVET does not appear to be affected on a consistent basis in cats treated for DCM (132). These values although still abnormal after the initiation of therapy, improve and move toward the normal range. Even when LVET is normal, gradual closure of the aortic valve may be seen as ventricular systolic pressures decline throughout systole and forward flow declines (Figure 4.95).
Figure 7.36 Pre-ejection periods are longer in hearts with dilated cardiomyopathy because it takes longer for systolic pressure to rise during isovolumic contraction (arrows). RA = right atrium, AO =

aorta, LA = left atrium.
The total systolic time, QAVC, measured from the onset of the QRS complex to closure of the aortic valve, is increased in cats with systolic dysfunction and dilated cardiomyopathy. This parameter consistently reflects increases in systolic function as cats with DCM are treated with inotropic drugs (132).
Mitral annular motion (MAM) of the septal mitral valve annulus obtained from apical four-chamber views reflects long-axis systolic function. Dogs with dilated cardiomyopathy have significantly decreased MAM, MAM%, and MAM index (Figure 7.37). Normal MAM is .46–1.74 cm while dogs with DCM have MAM ranging from .27–1.06 mm. The MAM%, a reflection of the contribution of annular motion to overall ventricular long-axis shortening, is 31–45% in normal dogs and 32–89% in dogs with DCM. MAM indexed to body surface area separates normal from abnormal dogs as well. MAM index is .78–3.78 cm/m2 in normal canine hearts and .16–1.27 cm/m2 in dogs with DCM. Mitral annular motion may be useful for the early detection of DCM in hearts where longitudinal shortening may be affected before radial shortening is affected (133).
Figure 7.37 Mitral annular motion (measurement) is depressed in a heart with systolic failure.

Early Diagnosis
Early dilated cardiomyopathy is a challenge to diagnose (129,134,135). The first signs of DCM may be cardiac arrhythmias, which include ventricular premature contractions and tachycardia, supraventricular tachycardia, or brady-arrhythmias (135,136). Echocardiographic exams in these animals typically reveal normal cardiac dimensions but very mild to moderately depressed fractional shortening (117,137). Diastolic left ventricular chamber size of greater than 46 mm, or a systolic chamber size of greater than 38 mm, or even one premature ventricular contraction suggests occult DCM in Doberman Pinschers (137). The interpretation of ventricular dilation and poor fractional shortening is sometimes difficult, especially when breed-specific echocardiographic reference ranges do not exist. In man, echocardiographic measurements of ventricular size must be greater than 112% of the predicted value (135,138,139). The progression of the disease is slow in most cases, and heart failure may not manifest itself for several years. Left ventricular chamber size will increase over the years as fractional shortening continues to deteriorate (90,117,136,137,140). Repeat echocardiographic exams are usually necessary to verify the diagnosis of DCM when subclinical disease exists.
Poor Fractional Shortening
Rule out poor preload as a cause.
Rule out increased afterload as a cause.
The European Society of Veterinary Cardiology has established criteria for the early diagnosis of DCM in the preclinical phase (135). A point system is used and applied to various echocardiographic and electrocardiographic parameters. Six points or greater is highly suggestive of the presence of subclinical DCM. Major criteria worth 3 points include left ventricular dilation in systole or diastole, increased sphericity (<1.65), fractional shortening less than 20–25% depending upon breed specific reference ranges, or an ejection fraction obtained using Simpson’s Rule from the right parasternal long-axis four-chamber view of less than 40%. Minor criteria worth 1 point include arrhythmia in Boxers and Dobermans, atrial fibrillation, increased E point to septal separation (EPSS), pre-ejection period to ejection time ratio >.4 (PEP/LVET), fractional shortening in the equivocal range (>20–25% but less than 30%), and left atrial or biatrial dilation. Other causes of myocardial failure should be

ruled out before the diagnosis of familial or idiopathic DCM is made. These criteria are listed in Table 7.4 (135).
Table 7.4
European Point System for the Diagnosis of Subclinical Dilated Cardiomyopathy
Major Criteria (3 points each) |
Minor Criteria (1 point each) |
|
|
Systolic or diastolic left ventricular dilation |
Arrhythmia if a Boxer or Doberman |
|
|
Increased sphericity (<1.65) |
Atrial fibrillation |
|
|
|
Increased EPSS (>7.7 mm) |
|
|
Fractional shortening (<20–25%) using breed specific values or ejection fraction <40% |
PEP/LVET >.4 |
|
|
|
Fractional shortening >20–25% but less than 30% |
|
|
|
Left atrial or biatrial dilation |
|
|
† = Significantly different from occult group.
Sphericity is assessed by comparing left ventricular length obtained from a four-chamber view to the M-mode measurement of diastolic dimension (Figure 7.38). Normal sphericity is 1.78 ± .16. Dilation develops and the chamber becomes more spherical as the ventricular chamber becomes dysfunctional (129,135).
Figure 7.38 The length of the left ventricular chamber in diastole is obtained from a four-chamber view. An apical view is used here; a parasternal four-chamber view may also be used. LV = left ventricle, VS = ventricular septum, RV = right ventricle, LA = left atrium, LVLd A4C = left ventricular length diastole apical four-chamber, LVEDV MOD A4C = left ventricular end diastolic volume method of discs apical four chamber.
Even using the European point system may lead to equivocal results. Reevaluation is often necessary and where there is no increase in ventricular chamber size or decrease in fractional shortening on subsequent echocardiographic exams, it indicates that the equivocal parameters of size and function may be normal for that specific animal (135). Fractional shortening has increased dramatically in man by as much as 10% in some patients, but it is generally transient (88). The same happens in many dogs, and fractional shortening may fluctuate from study to study.
Subclinical DCM

Using the European point system may help identify
6 points highly suggestive of subclinical DCM
Tissue Doppler imaging may be helpful in the early identification of dogs with dilated cardiomyopathy. Using right parasternal transverse images of the left ventricular chamber at the level of the papillary muscles, pulsed-wave color-tissue Doppler of radial motion shows decreased endocardial systolic and diastolic velocities when compared to epicardial velocity. A gradient is seen in dogs without heart disease, but the endocardial velocities in systole (Se′) and both phases of diastole (E′, A′) in dogs with subclinical DCM is lower (Figure 7.39). Radial epicardial velocities in systole and diastole are not different between normal dogs and dogs with subclinical DCM. This results in decreased radial trans myocardial velocity gradients (the difference between endocardial and epicardial velocities) during systole and early diastole (134,141,142). Longitudinal myocardial velocities of the left ventricular free wall from apical four-chamber views although still higher at the base than the apex as in normal dogs, are reduced at the base in systole and early diastole in dogs with subclinical DCM (142). The resulting longitudinal myocardial gradient (difference between basal and apical myocardial velocity) is reduced in systole and early diastole in dogs with preclinical dilated cardiomyopathy (Figure 7.40). Late diastolic radial and longitudinal myocardial gradients are not affected in the preclinical setting (134,142). The E′ : A′ ratio of radial and longitudinal myocardial velocities is also decreased in subclinical dilated cardiomyopathy (134,142).
Tissue Doppler and Subclinical DCM
Decreased epicardial to endocardial radial gradient
Decreased basal to apical longitudinal gradient
Figure 7.39 Decreased trans-myocardial radial velocity gradients (the difference between endocardial and epicardial velocities) are consistent with depressed systolic function. Two gates are placed on the left ventricular wall (arrow), and their myocardial velocity is displayed to the right (arrow). Velocity at the epicardium and endocardium are very similar.
Figure 7.40 Longitudinal myocardial gradient (difference between basal and apical myocardial

velocity, arrows on 2D image on the left) is reduced in systole (arrows on TDI image to the right) in dogs with preclinical dilated cardiomyopathy.
Predictors of Outcome
The clinical course of these animals is extremely variable, and the degree of ventricular dilation, the EPSS, or fractional shortening has no predictive value for survival time (120,143,144). Although 50% of dogs in one study survived past 3 months, M-mode echocardiographic parameters could not predict which dogs would be long-term survivors and which would not. In those studies, only the presence of pleural effusion, pulmonary edema, ascites, or a young age at diagnosis was found to suggest poorer survival times (143–146). Measurement of ejection fraction using the area length method on apical or right parasternal two-dimensional four-chamber imaging planes is a predictor of outcome and survival time (Figure 7.41). An ejection fraction <25% was a negative predictor with median survival time of 114 days (143). Calculation of the systolic volume index from M-mode using the Teicholz equation and the dog’s body surface area is also predictive of survival time (Figure 7.42). Body surface area tables derived from weight are in the appendix. Dogs with a systolic volume index >140 ml/m2 have a median survival time of 208 days (143).
Figure 7.41 Measurement of ejection fraction using apical or right parasternal two-dimensional fourchamber imaging planes is a predictor of outcome and survival time. (A) diastole, (B) systole. The ejection fraction here is 30%. LVLd A4C = left ventricular length diastole apical four chamber, LVEDV MOD A4C = left ventricular end diastolic volume method of discs apical four chamber, LVLs A4C = left ventricular length systole apical four chamber, LVESV MOD A4C = left ventricular end systolic volume method of discs apical four chamber, LVEF = left ventricular ejection fraction, SV MOD A4C = stroke volume method of discs apical four chamber, LV = left ventricle, RA = right atrium, LA = left atrium, RV = right ventricle.

Figure 7.42 Calculation of the systolic volume index from M-mode using the Teicholz volume calculation from an M-mode image and the dog’s body surface area. The diastolic volume here is 296 ml. This is divided by the dog’s body surface area.

Elevated left atrial pressure affects transmitral valve flow into the left ventricular chamber. When the increased left ventricular filling pressure results in a restrictive inflow pattern with a high E wave, rapid early deceleration and a lower A wave, it is associated with poor survival in dogs and in man with dilated cardiomyopathy. A transmitral E wave deceleration time of less than 80 msec correlated with poor survival in dogs (Figure 7.43) (143,147).
Rapid transmitral valve flow deceleration time (<80 msec) correlates with high LA pressures and a poor prognosis in dogs with DCM.
Figure 7.43 Rapid early mitral valve deceleration time (<80 msec) and a lower A wave velocity are associated with poor survival in dogs with dilated cardiomyopathy. MV DecT = mitral valve deceleration time.
The presence of pulmonary hypertension and concomitant poor right ventricular function is associated with a poorer prognosis for human patients with dilated cardiomyopathy (148,149). Eightynine percent of patients with hypertension were dead or hospitalized after 2 years compared to 32% of cardiomyopathy patients without pulmonary hypertension (149). In man, tricuspid annular motion of less than 1.25 cm is an independent predictor of poor survival (150).
There is currently no report of prognostic indicators for survival time since the advent of pimobendan therapy. The prognostic value of these parameters may be altered as more information is gathered in dogs receiving pimobendan.
Other Causes of Poor Systolic Function
When taurine deficiency is thought to be the underlying cause of DCM in cats, improvement in cardiac size and function occurs anywhere from 3 to 16 weeks after supplementation (151). These cats had essentially normal echocardiograms 6 months to 1 year after initial presentation (151).
The usually reversible systolic dysfunction found in dogs with hypothyroidism does not manifest itself with any significant dilation of the left ventricular chamber during diastole. Echocardiographic features in these hypothyroid dogs include increased systolic dimensions, decreased fractional shortening and Vcf, as well as increased PEP. Dogs with hypothyroidism and impaired function do not
typically show signs of heart failure unless pre-existing myocardial failure exists (93,152).
Sepsis can cause reversible myocardial failure and has been reported in the dog (153). Findings include dilation, decreased fractional shortening, secondary mitral regurgitation, and large end systolic volume indices. Myocardial dysfunction is seen in 40–50% of people with sepsis (154). Myocardial depression is thought to be secondary to circulating myocardial depressant factors, nitric oxide, tissue hypoxia, reperfusion injury, and increased circulating catecholamines (154–156).
Cancer treatment with doxorubicin is a documented cause of dilated cardiomyopathy. There is early depression in systolic function that occurs within the first few days after administration. This is reversible. With cumulative doses however, there are irreversible changes in systolic function. Echocardiographic changes include diastolic dysfunction early in the course of myocardial failure. Doppler echo shows increased isovolumic relaxation times and decreased early left ventricular filling. Global systolic failure occurs later. The septum in man is usually more affected than the rest of the left ventricle. Left ventricular dilation if present is mild, a feature that differentiates it from idiopathic dilated cardiomyopathy and most other causes of left-sided congestive heart failure (157).
Color Doppler Evaluation
Mitral insufficiency is common in animals with DCM secondary to dilation of the mitral annulus. The insufficiency varies from mild to severe when present and contributes to the clinical signs of congestive heart failure. Valvular lesions will not be evident if the insufficiency is truly secondary to the dilation.
Spectral Doppler Evaluation
Systolic Function
Maximal aortic flow velocity is typically reduced in animals with DCM (Figure 7.44) (158,159). Aortic flow velocities that remain within the normal range suggest maintenance of adequate cardiac output. Acceleration rate (dv/dt) may be a better indicator, but there is tremendous variation in this parameter in both healthy and DCM dogs (158,160).
Figure 7.44 Aortic flow velocities are reduced in animals with poor cardiac output. This flow velocity of 68 cm/sec was recorded in a dog with dilated cardiomyopathy.

Diastolic Function
Diastolic dysfunction may have a more significant impact on clinical signs than systolic failure. People and dogs with extremely poor systolic function often do not show significant clinical symptoms (161). The progression of occult to overt dilated cardiomyopathy may develop as ventricular filling is altered causing left atrial dilation and elevated left atrial pressure (158,161–163). Table 7.5 summarizes the diastolic parameters that change in the presence of dilated cardiomyopathy.
Table 7.5
Diastolic Function in Dogs with Dilated Cardiomyopathy
* = Significantly different from normal group.

† = Significantly different from occult group.
1 . O’Sullivan M, O’Grady M, Minors S. Assessment of diastolic function by Doppler echocardiography in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. Journal of Veterinary Internal Medicine 2007;21:81–91.
2 . Garncarz MA. Echocardiographic evaluation of diastolic parameters in dogs with dilated cardiomyopathy. Pol J Vet Sci 2007;10:207–215.
Mitral inflow studies in dogs with overt DCM show increased E wave velocity, no change in A wave velocity, increased E wave duration, an increased E : A ratio and decreased E wave deceleration time (Figures 4.75, 7.45) (161,162). Dogs with occult DCM have variable and inconsistent transmitral flow patterns probably reflecting different stages of disease progression. The presence of moderate to severe mitral insufficiency will also increase E velocity and must be considered when evaluating diastolic function and atrial pressures (Figure 4.81).
Figure 7.45 Doppler evaluation of left ventricular inflow may show increased E velocity, normal A velocity, and an increased E : A ratio secondary to increased atrial pressures and reduced ventricular compliance in hearts with dilated cardiomyopathy. Species = feline.
Changes in the mitral inflow profile in man are closely correlated with the improvement or deterioration of clinical status. An increase in E : A ratio signified deterioration of clinical status while a decrease in E : A ratio is observed as signs of heart failure resolve. These changes correlated to changes in capillary wedge pressure (163).
Rapid deceleration times (<180 msec) after peak E velocity are highly sensitive and specific for high mean left atrial pressure in man. Doppler evaluation of transmitral flow appears to be the best predictor of survival time in dogs at this point. A restrictive transmitral flow pattern (E : A ratio >2.0) and a rapid early diastolic deceleration (<80 msec) are associated with a median survival time of 114 days (Figure 7.43) (143). These predictive factors were true regardless of the presence of atrial fibrillation when just deceleration time was used for statistical analysis. A restrictive filling pattern represents high left atrial pressure and a rapid equilibration of ventricular and atrial pressure in the presence of reduced left ventricular compliance (143,162,164,165).

Diastolic pulmonary venous flow velocity in Doberman Pinchers with DCM is decreased. This occurs secondary to impaired relaxation, a slower decline in left ventricular pressure, and a slower rate of left atrial emptying. An S : D ratio greater than 1.0 will be seen. Decreased compliance is seen in later stages of this disease. Systolic pulmonary venous flow becomes low as left atrial pressure remains high during this phase of the cardiac cycle. Diastolic flow becomes more predominant as flow into the ventricle is enhanced with the elevated left atrial pressure and passive flow into the left atrium via the veins is enhanced. The S : D ratio becomes less than 1.0 (162). Velocity and duration of reverse diastolic flow (pulmonary vein A wave) late in diastole are both increased in man, but this has been difficult to show in dogs due to technical difficulties in obtaining these flow profiles (12).
Isovolumic relaxation time in dogs with overt DCM and in some dogs with subclinical DCM is shorter than normal. This is because increased left atrial pressure causes the mitral valve to open sooner aborting the relaxation time period. This is consistent with restrictive physiology as the disease progresses (161).
Pulsed-wave tissue Doppler evaluation of systolic myocardial motion at the left ventricular free wall mitral annular level shows reduced velocity from normal. Dobermans with overt cardiomyopathy have lower Sm velocity than those with occult DCM. This is a relatively early predictor in man for the presence of DCM but has only been reported in one canine study to date (142,162).
Early diastolic myocardial motion is reduced in some Dobermans with overt cardiomyopathy but is a consistent pattern in humans with DCM. The Doberman population was small and further study is warranted. The S′ velocity was consistently decreased in dogs with overt DCM however. This is a reflection of decreased compliance and restrictive physiology. A′ velocity decreased progressively from occult to overt disease (162).
Tissue Doppler and DCM
PW
S′ decreased at lateral mitral annulus
E′ may be decreased
Color TDI
Reduced radial and longitudinal gradients
Perhaps dyssynchrony between wall and septum
Color-tissue Doppler studies in Golden Retrievers with muscular dystrophy dilated cardiomyopathy show decreased radial endocardial systolic and diastolic velocities. Epicardial velocity did not change resulting in decreased trans-myocardial velocity gradients in these dogs with DCM. Longitudinal velocity of the left ventricular free wall was decreased at the annulus in systole and diastole, also resulting in a decreased gradient between the base and the apex (166).
Dogs with dilated cardiomyopathy have a strong negative relationship between the timing of systolic color-tissue Doppler motion with respect to the beginning of electrical systole and ejection fraction. The parameters are heart rate corrected by dividing each parameter by the square root of the R-to-R interval. Heart rate corrected parameters that are longer in dogs with DCM and that are negatively correlated with ejection fraction include the times from Q to start S′, Q to end S′ at the base of both the left ventricular free wall and septum (167). These changes are consistent with delays in longitudinal shortening of the left ventricular myocardium in DCM (167).
There is also a significant positive correlation between S′ velocity at the base of the free wall and ejection fraction. Peak velocity is depressed in dogs with dilated cardiomyopathy and may be an early indicator of disease (167,168). Breed variations exist, and this parameter needs further study in
